Phase-encoded fMRI tracks down brainstorms of natural language processing with subsecond precision
- PMID: 38339788
- PMCID: PMC10858339
- DOI: 10.1002/hbm.26617
Phase-encoded fMRI tracks down brainstorms of natural language processing with subsecond precision
Abstract
Natural language processing unfolds information overtime as spatially separated, multimodal, and interconnected neural processes. Existing noninvasive subtraction-based neuroimaging techniques cannot simultaneously achieve the spatial and temporal resolutions required to visualize ongoing information flows across the whole brain. Here we have developed rapid phase-encoded designs to fully exploit the temporal information latent in functional magnetic resonance imaging data, as well as overcoming scanner noise and head-motion challenges during overt language tasks. We captured real-time information flows as coherent hemodynamic waves traveling over the cortical surface during listening, reading aloud, reciting, and oral cross-language interpreting tasks. We were able to observe the timing, location, direction, and surge of traveling waves in all language tasks, which were visualized as "brainstorms" on brain "weather" maps. The paths of hemodynamic traveling waves provide direct evidence for dual-stream models of the visual and auditory systems as well as logistics models for crossmodal and cross-language processing. Specifically, we have tracked down the step-by-step processing of written or spoken sentences first being received and processed by the visual or auditory streams, carried across language and domain-general cognitive regions, and finally delivered as overt speeches monitored through the auditory cortex, which gives a complete picture of information flows across the brain during natural language functioning. PRACTITIONER POINTS: Phase-encoded fMRI enables simultaneous imaging of high spatial and temporal resolution, capturing continuous spatiotemporal dynamics of the entire brain during real-time overt natural language tasks. Spatiotemporal traveling wave patterns provide direct evidence for constructing comprehensive and explicit models of human information processing. This study unlocks the potential of applying rapid phase-encoded fMRI to indirectly track the underlying neural information flows of sequential sensory, motor, and high-order cognitive processes.
Keywords: brainstorms; dual-stream models; hemodynamic traveling waves; information flows; logistics models.
© 2024 The Authors. Human Brain Mapping published by Wiley Periodicals LLC.
Conflict of interest statement
Authors declare that they have no competing interests.
Figures


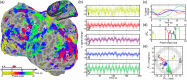
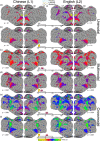
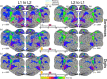

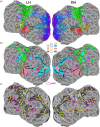



Update of
-
Phase-encoded fMRI tracks down brainstorms of natural language processing with sub-second precision.bioRxiv [Preprint]. 2023 May 29:2023.05.29.542546. doi: 10.1101/2023.05.29.542546. bioRxiv. 2023. Update in: Hum Brain Mapp. 2024 Feb 1;45(2):e26617. doi: 10.1002/hbm.26617. PMID: 37398177 Free PMC article. Updated. Preprint.
References
-
- Atkinson, R. C. , & Shiffrin, R. M. (1968). Human memory: A proposed system and its control processes. In Spence K. W. & Spence J. T. (Eds.), Psychology of learning and motivation: Advances in research and theory (Vol. 2, pp. 89–195). Academic Press. 10.1016/S0079-7421(08)60422-3 - DOI
-
- Bolt, T. , Nomi, J. S. , Bzdok, D. , Salas, J. A. , Chang, C. , Thomas Yeo, B. T. , Uddin, L. Q. , & Keilholz, S. D. (2022). A parsimonious description of global functional brain organization in three spatiotemporal patterns. Nature Neuroscience, 25(8), 1093–1103. 10.1038/s41593-022-01118-1 - DOI - PubMed
-
- Buchsbaum, B. R. , Hickok, G. , & Humphries, C. (2001). Role of left posterior superior temporal gyrus in phonological processing for speech perception and production. Cognitive Science, 25(5), 663–678. 10.1016/S0364-0213(01)00048-9 - DOI
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

