Proton Pump Inhibitors and Cyclin-Dependent Kinase 4/6 Inhibitors in Patients With Breast Cancer
- PMID: 38340010
- PMCID: PMC11144975
- DOI: 10.1093/oncolo/oyae015
Proton Pump Inhibitors and Cyclin-Dependent Kinase 4/6 Inhibitors in Patients With Breast Cancer
Abstract
Background: Proton pump inhibitors (PPIs) reduce the bioavailability of several anticancer drugs. The impact of PPIs co-administered with cyclin-dependent kinase 4 and 6 inhibitors is controversial. We aimed to clarify whether the concomitant use of PPIs impacts palbociclib and abemaciclib effectiveness in breast cancer treatment.
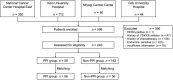
Patients and methods: This multicenter, retrospective, observational study, conducted across 4 medical institutions in Japan, consecutively included patients with endocrine-resistant metastatic breast cancer, receiving palbociclib or abemaciclib between December 2017 and August 2022. Propensity score-matched analyses were performed. Treatment efficacy and safety with and without PPIs were compared. Progression-free survival and overall survival were estimated using the Kaplan-Meier method and compared using a log-rank test. A Cox proportional hazards model was used to estimate the hazard ratio.
Results: The study included 240 patients. After 1:1 matching, 112 patients were treated with and without PPIs. The median progression-free survival period was 1.2 years in the PPI group and 1.3 years in the non-PPI group (hazard ratio, 1.19; 95% CI, 0.70-2.02). The median overall survival period was 3.6 years in the PPI group, whereas it was not reached in the non-PPI group (hazard ratio, 1.23; 95% CI, 0.61-2.47). Consistent results were obtained for subgroups receiving palbociclib (n = 177) and abemaciclib (n = 63) without propensity score matching. Adverse event incidence and severity were similar in both groups.
Conclusion: The effectiveness of cyclin-dependent kinase 4/6 inhibitors is unlikely to be affected by concomitant PPI use. Future prospective pharmacokinetic studies are warranted.
Keywords: abemaciclib; breast cancer; palbociclib; propensity score; proton pump inhibitor.
© The Author(s) 2024. Published by Oxford University Press.
Conflict of interest statement
R.U. received personal fees from Eisai, Sawai Pharmaceutical, SBI Pharmaceuticals, Daiichi Sankyo, Statcom, and EPS Corporation, and lecture fees from Janssen Pharmaceutical and SAS Institute Japan outside the submitted work. T.M. received grants from Sysmex, Eisai, MSD, Pfizer, Novartis, Chugai, AstraZeneca, Ono, Daiichi Sankyo, and Gilead Sciences and lecture fees from Eisai, Pfizer, Novartis, Chugai, Eli Lilly, AstraZeneca, Kyowa Kirin, Taiho, and Daiichi Sankyo outside the submitted work. T.H. received a research grant from Eli Lilly and lecture fees from Eli Lilly and Pfizer outside the submitted work. H.I. received consulting fees from Taiho, consulting fees provided to the institution from Eisai and Taiho, and lecture fees from Astellas, AstraZeneca, Chugai, Daiichi Sankyo, Eli Lilly, Nippon Boehringer Ingelheim, Nippon Kayaku, Ono, Taiho, and Yakult outside the submitted work. K.K. received payment for presentations from Daiichi Sankyo and Merck Serono, and payment for expert testimony from Daiichi Sankyo outside the submitted work. Y.K. received grants provided to the institution from Asahi Kasei, Ono, Otsuka, Nippon Covidien, Taiho, Chugai, Kaken, EA Pharma, Yakult, Otsuka, Tsumura, Sumitomo, Eisai, Kyowa Kirin, Takeda, Teijin, Cardinal Health, and Kowa, and personal fees from Asahi Kasei, AstraZeneca, Ethicon, Ono, Otsuka, Olympus, Cardinal Health, Shionogi, Taiho, Chugai, Bristol-Myers Squibb, MSD, Smith & Nephew, Kaken, Aska, Miyarisan, Toray Industries, Daiichi Sankyo, Chugai Foundation for Innovative Drug Discovery Science, Nippon Kayaku, EA Pharma, Intuitive Surgical, Takeda, Sysmex, and Tsumura, and joint research laboratory for development and education of innovative medical technology of Sysmex and Medicaroid corporation outside the submitted work. M.K. received a lecture fee from Eisai outside the submitted work. A.N. received lecture fees from Chugai, Pfizer, and Daiichi Sankyo and owns stock of Chugai. A.S. received grants provided to the institution from Nippon Kayaku, Asahi Kasei, Chugai, Taiho, Daiichi Sankyo, Japan Blood Products Organization, Mochida, and Sun Pharma, and lecture fees from Toa Eiyo, Asahi Kasei, Daiichi Sankyo, Pfizer, Eisai, Nippon Shinyaku, Celltrion Healthcare Japan, Otsuka, Sandoz, Tsumura, Nipro, Taiho, Kyowa Kirin, Nippon Chemiphar, Japan Blood Products Organization, Takeda, and Nippon Boehringer Ingelheim outside the submitted work. M.F. received lecture fees from Daiichi Sankyo, Taiho, Chugai, Eisai, Eli Lilly, and Nippon Kayaku outside the submitted work. H.K. received research funding from Eli Lilly outside the submitted work. T.N. received research funding from Chugai, Daiichi Sankyo, Kyowa Kirin, Otsuka, Sanofi, and Shionogi outside the submitted work. The other authors declared no Conflict of Interest. The aforementioned funders had no role in the design, conduct, or reporting of this study.
Figures

Similar articles
-
Concomitant Use of Proton Pump Inhibitors and CDK4/6 Inhibitors in Metastatic Hormone-Positive Breast Cancer: A Real-World Cohort Study.Oncology. 2025;103(6):498-507. doi: 10.1159/000542693. Epub 2024 Nov 25. Oncology. 2025. PMID: 39586283
-
Cyclin-dependent kinase 4/6 inhibitors for the management of advanced or metastatic breast cancer in women.Am J Health Syst Pharm. 2019 Aug 1;76(16):1183-1202. doi: 10.1093/ajhp/zxz121. Am J Health Syst Pharm. 2019. PMID: 31369120 Review.
-
Comparison of treatment-related adverse events of different Cyclin-dependent kinase 4/6 inhibitors in metastatic breast cancer: A network meta-analysis.Cancer Treat Rev. 2020 Nov;90:102086. doi: 10.1016/j.ctrv.2020.102086. Epub 2020 Aug 17. Cancer Treat Rev. 2020. PMID: 32861975
-
Comparison of Safety and Treatment Continuity of Palbociclib and Abemaciclib for Hormone Receptor-Positive, HER2-Negative Metastatic/Recurrent Breast Cancer.J Pharm Pract. 2024 Dec;37(6):1258-1266. doi: 10.1177/08971900241247653. Epub 2024 Apr 23. J Pharm Pract. 2024. PMID: 38652858
-
Role of cyclin-dependent kinase 4/6 inhibitors in the current and future eras of cancer treatment.J Oncol Pharm Pract. 2019 Jan;25(1):110-129. doi: 10.1177/1078155218770904. Epub 2018 May 4. J Oncol Pharm Pract. 2019. PMID: 29726787 Review.
Cited by
-
Efficacy of cyclin-dependent kinase inhibitors with concurrent proton pump inhibitors in patients with breast cancer: a systematic review and meta-analysis.Oncologist. 2025 Feb 6;30(2):oyae320. doi: 10.1093/oncolo/oyae320. Oncologist. 2025. PMID: 39963828 Free PMC article.
References
-
- Cancer Statistics in Japan; Cancer Information Services, Cancer type statistical information Breast. Japan: National Cancer Center. https://ganjoho.jp/reg_stat/statistics/stat/cancer/14_breast.html [in Japanese]. Accessed November 22, 2022.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

