Monoclonal antibodies targeting the calcitonin gene-related peptide pathway improve the effectiveness of acute medication-a real-world study
- PMID: 38340218
- PMCID: PMC11176241
- DOI: 10.1007/s10072-024-07380-4
Monoclonal antibodies targeting the calcitonin gene-related peptide pathway improve the effectiveness of acute medication-a real-world study
Abstract
Background: One of the aims of migraine prevention is to improve response to acute migraine treatments. The aim of the present study was to assess whether monoclonal antibodies targeting the CGRP pathway (CGRP-mAbs) can improve the perceived efficacy of acute treatments.
Methods: We included and followed up patients with chronic or episodic migraine from the Headache Centers of Avezzano-L'Aquila and Naples treated with CGRP-mAbs from March 2021 to December 2022. All patients filled out the Migraine Treatment Optimization Questionnaire (MTOQ), the Headache Impact Test (HIT-6), and the Migraine Impact and Disability Assessment Scale (MIDAS) at baseline and 3-6 months after the start of treatment with CGRP-mAbs.
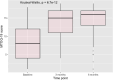
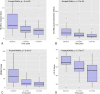
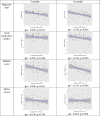
Results: Sixty-five patients (81.3%) completed the 6-month follow-up. Most patients were female (55, 84.6%), with a median age of 46 years (IQR 39-56). Median MTOQ score increased from 8 (interquartile range [IQR] 4-13) at baseline to 15 (IQR 11-17) at 3 months (p < 0.001) and 16 (IQR 13-17) at the 6-month follow-up (p < 0.001). Median migraine days over 90-day periods decreased from 40 (IQR 24-60) to 24 (IQR 15-30) at 3 months (p < 0.001) and to 20 (IQR 12-24) at 6 months (p < 0.001). Median monthly intake of acute medication decreased from 55 doses (IQR 29-80.5) to 24 doses (IQR 15-40) at 3 months and 18 doses (IQR 11-30) at 6 months (p < 0.001).
Conclusions: We showed that 6 months of preventive treatment with CGRP-mAbs led to a significantly better effectiveness of acute treatments, paralleled by decreased monthly migraine days and acute treatment intake.
Keywords: Acute medication; Calcitonin gene-related peptide; Migraine; Migraine treatment.
© 2024. The Author(s).
Conflict of interest statement
V.C. has received honoraria for participation in advisory boards sponsored by Novartis and speaker honoraria sponsored by Teva. M.S. has received speaker honoraria from Novartis, Teva, and Lilly. A.R. has received speaker honoraria from Allergan, Lilly, Novartis, Biogen, and Teva and serves as an associate editor of Frontiers in Neurology (Headache Medicine and Facial Pain session). S.S. reports personal fees as speaker or advisor by Abbott, Allergan-Abbvie, AstraZeneca, Boehringer, Eli Lilly, Lundbeck, Novartis, Novo Nordisk, Pfizer, Teva; research grants by Novartis, Uriach; president-elect European Stroke Organisation, second vice president of the European Headache Federation, specialty chief editor in Headache and Neurogenic Pain for Frontiers in Neurology, associate editor for the Journal of Headache and Pain, assistant editor for Stroke. RO reports personal fees from Novartis, Teva, Lilly, and Pfizer and non-financial support from AbbVie/Allergan, Lilly, Novartis, and Teva. The other authors have nothing to declare.
Figures
Similar articles
-
Anti‑CGRP monoclonal antibodies in resistant migraine: preliminary real-world effectiveness and clinical predictors of response at two years.Int J Clin Pharm. 2024 Dec;46(6):1317-1326. doi: 10.1007/s11096-024-01758-2. Epub 2024 Jul 11. Int J Clin Pharm. 2024. PMID: 38990457
-
Persistence, effectiveness, and tolerability of anti-calcitonin gene-related peptide monoclonal antibodies in patients with chronic migraine.Headache. 2025 Jan;65(1):24-34. doi: 10.1111/head.14827. Epub 2024 Sep 13. Headache. 2025. PMID: 39268992
-
Effectiveness of switching strategies in CGRP monoclonal antibody therapy for migraine: A retrospective cohort study.Headache. 2025 Apr;65(4):619-630. doi: 10.1111/head.14865. Epub 2024 Dec 27. Headache. 2025. PMID: 39727075 Free PMC article.
-
Indirect Comparison of Topiramate and Monoclonal Antibodies Against CGRP or Its Receptor for the Prophylaxis of Episodic Migraine: A Systematic Review with Meta-Analysis.CNS Drugs. 2021 Aug;35(8):805-820. doi: 10.1007/s40263-021-00834-9. Epub 2021 Jul 16. CNS Drugs. 2021. PMID: 34272688 Free PMC article.
-
Long-Term Outcome After Discontinuation of CGRP-Targeting Therapy for Migraine.Curr Pain Headache Rep. 2024 Aug;28(8):743-751. doi: 10.1007/s11916-024-01259-x. Epub 2024 Apr 29. Curr Pain Headache Rep. 2024. PMID: 38683278 Review.
References
-
- Marmura MJ, Diener HC, Cowan RP, Tepper SJ, Diamond ML, Starling AJ, et al. Preventive migraine treatment with eptinezumab reduced acute headache medication and headache frequency to below diagnostic thresholds in patients with chronic migraine and medication-overuse headache. Headache. 2021;61:1421–1431. doi: 10.1111/head.14206. - DOI - PMC - PubMed
-
- Ambrosini A, Estemalik E, Pascual J, Rettiganti M, Stroud C, Day K, et al. Changes in acute headache medication use and health care resource utilization: results from a randomized, double-blind, placebo-controlled clinical trial evaluating galcanezumab in adults with treatment-resistant migraine (CONQUER) J Manag Care Spec Pharm. 2022;28:645–656. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

