Prognostic value of circulating short-length DNA fragments in unresected glioblastoma patients
- PMID: 38340682
- PMCID: PMC10867437
- DOI: 10.1016/j.tranon.2024.101897
Prognostic value of circulating short-length DNA fragments in unresected glioblastoma patients
Abstract
Background: Liquid biopsy application is still challenging in glioblastoma patients and the usefulness of short-length DNA (slDNA) fragments is not established. The aim was to investigate slDNA concentration as a prognostic marker in unresected glioblastoma patients.
Methods: Patients with unresected glioblastoma and treated by radiochemotherapy (RT/TMZ) were included. Plasmas were prospectively collected at three times: before (pre-) RT, after (post-) RT and at the time of progression. Primary objective was to investigate the impact on survival of slDNA concentration [slDNA] variation during RT/TMZ. Secondary objectives were to explore the association between tumor volume, corticosteroid exposition and [slDNA]; and the impact of slDNA detection at pre-RT on survival.
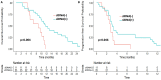
Results: Thirty-six patients were analyzed: 11 patients (30.6 %) experienced [slDNA] decrease during RT/TMZ, 22 patients (61.1 %) experienced increase and 3 patients (8.3 %) had stability. Decrease of [slDNA] during RT/TMZ was associated with better outcome compared to increase or stability: median OS, since end of RT, of 13.2 months [11.4 - NA] vs 10.1 months [7.8 - 12.6] and 6.8 months [4.5 - NA], p = 0.015, respectively. slDNA detection at pre-RT time was associated with improved OS: 11.7 months in the slDNA(+) group versus 8.8 months in the slDNA(-) group, p = 0.004. [slDNA] was not associated with corticosteroids exposition or tumor volume. No influence on survival was observed for both whole cfDNA concentration or slDNA peak size.
Conclusion: [slDNA] decrease during radiochemotherapy phase is a favorable prognostic marker on OS for unresected glioblastoma patients. Larger and independent cohorts are now required.
Trial registration: ClinicalTrial, NCT02617745. Registered 1 December 2015, https://clinicaltrials.gov/ct2/show/NCT02617745?term=glioplak&draw=2&rank=1.
Keywords: Glioblastoma; Liquid biopsy; Plasma; Prognostic; Short-length DNA.
Copyright © 2024. Published by Elsevier Inc.
Conflict of interest statement
Declaration of competing interest MF declares income received for research purposes from Servier® company, benefits for interventions from Seagen® and Novocure®, and payment of congress fees from Gilead® and Pfizer®. FC declares benefits for interventions from BMS®, Merck Serono®, MSD®, Gilead®, Astra Zeneca® and Novartis®, and payment of congress fees from Novartis®, Pfizer®, Merck® and Nutricia®. All these conflicts are outside the field of the submitted work. The other authors declare no conflict of interest.
Figures




References
-
- Wen P.Y., Weller M., Lee E.Q., Alexander B.M., Barnholtz-Sloan J.S., Barthel F.P., et al. Glioblastoma in adults: a Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions. Neuro-Oncol. Août. 2020;22(8):1073‑113. - PMC - PubMed
-
- Garcia-Murillas I., Schiavon G., Weigelt B., Ng C., Hrebien S., Cutts R.J., et al. Mutation tracking in circulating tumor DNA predicts relapse in early breast cancer. Sci. Transl. Med. 2015;7(302):302ra133. 26 août. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

