Identification of a core transcriptional program driving the human renal mesenchymal-to-epithelial transition
- PMID: 38340720
- PMCID: PMC7616043
- DOI: 10.1016/j.devcel.2024.01.011
Identification of a core transcriptional program driving the human renal mesenchymal-to-epithelial transition
Abstract
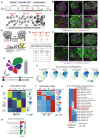
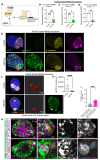
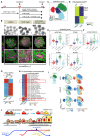
During kidney development, nephron epithelia arise de novo from fate-committed mesenchymal progenitors through a mesenchymal-to-epithelial transition (MET). Downstream of fate specification, transcriptional mechanisms that drive establishment of epithelial morphology are poorly understood. We used human iPSC-derived renal organoids, which recapitulate nephrogenesis, to investigate mechanisms controlling renal MET. Multi-ome profiling via snRNA-seq and ATAC-seq of organoids identified dynamic changes in gene expression and chromatin accessibility driven by activators and repressors throughout MET. CRISPR interference identified that paired box 8 (PAX8) is essential for initiation of MET in human renal organoids, contrary to in vivo mouse studies, likely by activating a cell-adhesion program. While Wnt/β-catenin signaling specifies nephron fate, we find that it must be attenuated to allow hepatocyte nuclear factor 1-beta (HNF1B) and TEA-domain (TEAD) transcription factors to drive completion of MET. These results identify the interplay between fate commitment and morphogenesis in the developing human kidney, with implications for understanding both developmental kidney diseases and aberrant epithelial plasticity following adult renal tubular injury.
Keywords: epithelial polarity; kidney development; mesenchymal-to-epithelial transition; organoids; transcriptional regulation.
Copyright © 2024 MRC Laboratory of Molecular Biology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests J.M.W. is an employee and stockholder of AstraZeneca.
Figures





References
-
- Gilmour D, Rembold M, Leptin M. From morphogen to morphogenesis and back. Nature. 2017;541:311–320. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

