Keratinocyte integrin α3β1 induces expression of the macrophage stimulating factor, CSF-1, through a YAP/TEAD-dependent mechanism
- PMID: 38340968
- PMCID: PMC10923166
- DOI: 10.1016/j.matbio.2024.02.003
Keratinocyte integrin α3β1 induces expression of the macrophage stimulating factor, CSF-1, through a YAP/TEAD-dependent mechanism
Abstract
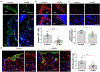
The development of wound therapy targeting integrins is hampered by inadequate understanding of integrin function in cutaneous wound healing and the wound microenvironment. Following cutaneous injury, keratinocytes migrate to restore the skin barrier, and macrophages aid in debris clearance. Thus, both keratinocytes and macrophages are critical to the coordination of tissue repair. Keratinocyte integrins have been shown to participate in this coordinated effort by regulating secreted factors, some of which crosstalk to distinct cells in the wound microenvironment. Epidermal integrin α3β1 is a receptor for laminin-332 in the cutaneous basement membrane. Here we show that wounds deficient in epidermal α3β1 express less epidermal-derived macrophage colony-stimulating factor 1 (CSF-1), the primary macrophage-stimulating growth factor. α3β1-deficient wounds also have fewer wound-proximal macrophages, suggesting that keratinocyte α3β1 may stimulate wound macrophages through the regulation of CSF-1. Indeed, using a set of immortalized keratinocytes, we demonstrate that keratinocyte-derived CSF-1 supports macrophage growth, and that α3β1 regulates Csf1 expression through Src-dependent stimulation of Yes-associated protein (YAP)-Transcriptional enhanced associate domain (TEAD)-mediated transcription. Consistently, α3β1-deficient wounds in vivo display a substantially reduced number of keratinocytes with YAP-positive nuclei. Overall, our current findings identify a novel role for epidermal integrin α3β1 in regulating the cutaneous wound microenvironment by mediating paracrine crosstalk from keratinocytes to wound macrophages, implicating α3β1 as a potential target of wound therapy.
Keywords: Csf-1; Integrin α3β1; Keratinocyte; Macrophage; Wound healing; YAP/TAZ.
Copyright © 2024 Elsevier B.V. All rights reserved.
Figures

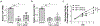


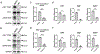

Similar articles
-
Keratinocyte Integrin α3β1 Promotes Efficient Healing of Wound Epidermis.JID Innov. 2024 Aug 29;5(1):100310. doi: 10.1016/j.xjidi.2024.100310. eCollection 2025 Jan. JID Innov. 2024. PMID: 39385750 Free PMC article.
-
Podocyte YAP ablation decreases podocyte adhesion and exacerbates FSGS progression through α3β1 integrin.J Pathol. 2025 Jan;265(1):84-98. doi: 10.1002/path.6370. J Pathol. 2025. PMID: 39668547
-
Downregulation of the metalloproteinases ADAM10 or ADAM17 promotes osteoclast differentiation.Cell Commun Signal. 2024 Jun 11;22(1):322. doi: 10.1186/s12964-024-01690-y. Cell Commun Signal. 2024. PMID: 38863060 Free PMC article.
-
Dressings and topical agents for treating pressure ulcers.Cochrane Database Syst Rev. 2017 Jun 22;6(6):CD011947. doi: 10.1002/14651858.CD011947.pub2. Cochrane Database Syst Rev. 2017. PMID: 28639707 Free PMC article.
-
Antibiotics and antiseptics for surgical wounds healing by secondary intention.Cochrane Database Syst Rev. 2016 Mar 29;3(3):CD011712. doi: 10.1002/14651858.CD011712.pub2. Cochrane Database Syst Rev. 2016. PMID: 27021482 Free PMC article.
Cited by
-
YAP/TAZ Signalling Controls Epidermal Keratinocyte Fate.Int J Mol Sci. 2024 Nov 30;25(23):12903. doi: 10.3390/ijms252312903. Int J Mol Sci. 2024. PMID: 39684613 Free PMC article. Review.
-
The epidermal integrin-mediated secretome regulates the skin microenvironment during tumorigenesis and repair.Matrix Biol. 2024 Dec;134:175-183. doi: 10.1016/j.matbio.2024.11.002. Epub 2024 Nov 2. Matrix Biol. 2024. PMID: 39491760 Review.
-
Roles for epithelial integrin α3β1 in regulation of the microenvironment during normal and pathological tissue remodeling.Am J Physiol Cell Physiol. 2024 May 1;326(5):C1308-C1319. doi: 10.1152/ajpcell.00128.2024. Epub 2024 Mar 18. Am J Physiol Cell Physiol. 2024. PMID: 38497112 Free PMC article. Review.
-
Keratinocyte Integrin α3β1 Promotes Efficient Healing of Wound Epidermis.JID Innov. 2024 Aug 29;5(1):100310. doi: 10.1016/j.xjidi.2024.100310. eCollection 2025 Jan. JID Innov. 2024. PMID: 39385750 Free PMC article.
References
-
- Hynes RO, Integrins: bidirectional, allosteric signaling machines. Cell, 2002. 110(6): p. 673–87. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

