Management of adult patients with podocytopathies: an update from the ERA Immunonephrology Working Group
- PMID: 38341276
- PMCID: PMC11024823
- DOI: 10.1093/ndt/gfae025
Management of adult patients with podocytopathies: an update from the ERA Immunonephrology Working Group
Erratum in
-
Correction to: Management of adult patients with podocytopathies: an update from the ERA Immunonephrology Working Group.Nephrol Dial Transplant. 2025 May 30;40(6):1260. doi: 10.1093/ndt/gfae215. Nephrol Dial Transplant. 2025. PMID: 39351629 Free PMC article. No abstract available.
Abstract
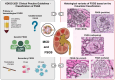
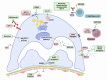
The histopathological lesions, minimal change disease (MCD) and focal segmental glomerulosclerosis (FSGS) are entities without immune complex deposits which can cause podocyte injury, thus are frequently grouped under the umbrella of podocytopathies. Whether MCD and FSGS may represent a spectrum of the same disease remains a matter of conjecture. Both frequently require repeated high-dose glucocorticoid therapy with alternative immunosuppressive treatments reserved for relapsing or resistant cases and response rates are variable. There is an unmet need to identify patients who should receive immunosuppressive therapies as opposed to those who would benefit from supportive strategies. Therapeutic trials focusing on MCD are scarce, and the evidence used for the 2021 Kidney Disease: Improving Global Outcomes (KDIGO) guideline for the management of glomerular diseases largely stems from observational and pediatric trials. In FSGS, the differentiation between primary forms and those with underlying genetic variants or secondary forms further complicates trial design. This article provides a perspective of the Immunonephrology Working Group (IWG) of the European Renal Association (ERA) and discusses the KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases focusing on the management of MCD and primary forms of FSGS in the context of recently published evidence, with a special emphasis on the role of rituximab, cyclophosphamide, supportive treatment options and ongoing clinical trials in the field.
Keywords: FSGS; KDIGO; MCD; guideline; podocytopathy.
© The Author(s) 2024. Published by Oxford University Press on behalf of the ERA.
Conflict of interest statement
S.M. received honoraria from Baxter, and support for meeting registration and travel from Abdi Ibrahim Otsuka, Amgen, Roche, and Sanofi Genzyme. L.D.-F. received research grants from Delta 4. I.M.B. consulted for Boehringer-Ingelheim, GSK, Novartis, Catalyst Biosciences, Toleranzia, Vera, and Hansa Biopharma and received educational grants from CSL Vifor. She is the owner of BiPath and on the BOD of the Renal Pathology Society. A.B. received consultancy fees and honoraria from Amgen, AstraZeneca, Bayer, Fresenius, and CSL Vifor. M.G. received an advisory board fee from Retrophin. K.I.S. received consultancy fees from Bayer and Boehringer Ingelheim. A.K. received consultancy fees from CSL Vifor, Otsuka, GSK, Walden Biosciences, Catalyst Biosciences, and Delta4; and received unrestricted research grants from CSL Vifor and Otsuka. S.M., E.F., S.S., and A.K. are editorial board members of the journal NDT. The remaining authors disclosed no conflicts of interest.
Figures