Facilitating long-term cell examinations and time-lapse recordings in cell biology research with CO2 mini-incubators
- PMID: 38341451
- PMCID: PMC10858865
- DOI: 10.1038/s41598-024-52866-y
Facilitating long-term cell examinations and time-lapse recordings in cell biology research with CO2 mini-incubators
Abstract
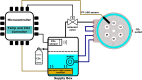
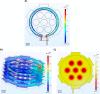
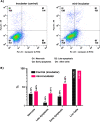
In recent years, microscopy has revolutionized the study of dynamic living cells. However, performing long-term live cell imaging requires stable environmental conditions such as temperature, pH, and humidity. While standard incubators have traditionally provided these conditions, other solutions, like stagetop incubators are available. To further enhance the accessibility of stable cell culture environments for live cell imaging, we developed a portable CO2 cell culture mini-incubator that can be easily adapted to any x-y inverted microscope stage, enabling long-term live cell imaging. This mini-incubator provides and maintains stable environmental conditions and supports cell viability comparable to standard incubators. Moreover, it allows for parallel experiments in the same environment, saving both time and resources. To demonstrate its functionality, different cell lines (VERO and MDA-MB-231) were cultured and evaluated using various assays, including crystal violet staining, MTT, and flow cytometry tests to assess cell adhesion, viability, and apoptosis, respectively. Time-lapse imaging was performed over an 85-h period with MDA-MB-231 cells cultured in the mini-incubator. The results indicate that this device is a viable solution for long-term imaging and can be applied in developmental biology, cell biology, and cancer biology research where long-term time-lapse recording is required.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
Establishment of the microscope incubation system and its application in evaluating tumor treatment effects through real-time live cellular imaging.Front Bioeng Biotechnol. 2024 Aug 16;12:1447265. doi: 10.3389/fbioe.2024.1447265. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 39219621 Free PMC article.
-
Open-dish incubator for live cell imaging with an inverted microscope.Biotechniques. 2003 Oct;35(4):708-14, 716. doi: 10.2144/03354bi01. Biotechniques. 2003. PMID: 14579735
-
Comparison of embryo implantation potential between time-lapse incubators and standard incubators: a randomized controlled study.Reprod Biomed Online. 2022 Nov;45(5):858-866. doi: 10.1016/j.rbmo.2022.06.017. Epub 2022 Jun 28. Reprod Biomed Online. 2022. PMID: 36210273 Clinical Trial.
-
Decisions for the IVF laboratory: comparative analysis of embryo culture incubators.Reprod Biomed Online. 2014 May;28(5):535-47. doi: 10.1016/j.rbmo.2014.01.004. Epub 2014 Jan 27. Reprod Biomed Online. 2014. PMID: 24656561 Review.
-
Choosing the best embryo by time lapse versus standard morphology.Fertil Steril. 2015 Feb;103(2):323-32. doi: 10.1016/j.fertnstert.2014.11.003. Epub 2014 Dec 17. Fertil Steril. 2015. PMID: 25527231 Review.
Cited by
-
Establishment of the microscope incubation system and its application in evaluating tumor treatment effects through real-time live cellular imaging.Front Bioeng Biotechnol. 2024 Aug 16;12:1447265. doi: 10.3389/fbioe.2024.1447265. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 39219621 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

