Reduction of prevalence of patients meeting the criteria for metabolic syndrome with tirzepatide: a post hoc analysis from the SURPASS Clinical Trial Program
- PMID: 38341541
- PMCID: PMC10859014
- DOI: 10.1186/s12933-024-02147-9
Reduction of prevalence of patients meeting the criteria for metabolic syndrome with tirzepatide: a post hoc analysis from the SURPASS Clinical Trial Program
Abstract
Background: Metabolic syndrome is characterized as the co-occurrence of interrelated cardiovascular risk factors, including insulin resistance, hyperinsulinemia, abdominal obesity, dyslipidemia and hypertension. Once weekly tirzepatide is approved in the US and EU for the treatment of type 2 diabetes (T2D) and obesity. In the SURPASS clinical trial program for T2D, tirzepatide demonstrated greater improvements in glycemic control, body weight reduction and other cardiometabolic risk factors versus placebo, subcutaneous semaglutide 1 mg, insulin degludec, and insulin glargine. This post hoc analysis assessed the effect of tirzepatide use on the prevalence of patients meeting the criteria for metabolic syndrome across SURPASS 1-5.
Methods: Metabolic syndrome was defined as having ≥ 3 of 5 criteria according to the US National Cholesterol Education Program: Adult Treatment Panel III. Analyses were based on on-treatment data at the primary endpoint from patients adherent to treatment (taking ≥ 75% study drug). A logistic regression model with metabolic syndrome status as the response variable, metabolic syndrome status at the baseline visit as an adjustment, and randomized treatment as fixed explanatory effect was used. The effect of tirzepatide use on the prevalence of patients meeting the criteria for metabolic syndrome by categorical weight loss, background medication and gender were assessed.
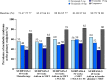
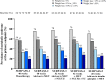
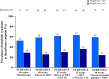
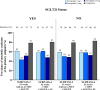
Results: In SURPASS, the prevalence of patients meeting the criteria for metabolic syndrome at baseline was 67-88% across treatment groups with reductions at the primary endpoint to 38-64% with tirzepatide versus 64-82% with comparators. Reductions in the prevalence of patients meeting the criteria for metabolic syndrome was significantly greater with all tirzepatide doses versus placebo, semaglutide 1 mg, insulin glargine, and insulin degludec (p < 0.001). Individual components of metabolic syndrome were also reduced to a greater extent with tirzepatide vs comparators. Greater reductions in body weight were associated with greater reductions in the prevalence of patients meeting the criteria for metabolic syndrome and its individual components. Background SGLT2i or sulfonylurea use or gender did not impact the change in prevalence of patients meeting the criteria for metabolic syndrome.
Conclusions: In this post hoc analysis, tirzepatide at all doses studied was associated with a greater reduction in the prevalence of patients meeting the criteria for metabolic syndrome compared to placebo, semaglutide 1 mg, insulin degludec, and insulin glargine. Although more evidence is needed, these data would support greater potential improvement in cardiovascular risk factor profile with tirzepatide treatment in people across the continuum of T2D.
Keywords: Incretin; Metabolic syndrome; Tirzepatide; Type 2 diabetes.
© 2024. The Author(s).
Conflict of interest statement
SJN has received research support from AstraZeneca, New Amsterdam Pharma, Amgen, Anthera, Eli Lilly and Company, Esperion, Novartis, Cerenis, The Medicines Company, Resverlogix, InfraReDx, Roche, Sanofi-Regeneron and LipoScience and is a consultant for AstraZeneca, Amarin, Akcea, Eli Lilly and Company, Anthera, Omthera, Merck, Takeda, Resverlogix, Sanofi-Regeneron, CSL Behring, Esperion, Boehringer Ingelheim and Vaxxinity. ST has served on advisory boards and received consulting fees from Boehringer Ingelheim, Eli Lilly and Company, Johnson and Johnson, Novo Nordisk, and Sanofi. CWlR serves on advisory boards of Novo Nordisk A/S, Herbalife, GI Dynamics, Eli Lilly and Company, Johnson & Johnson, Glia, and Boehringer Ingelheim and was gifted stockholdings in September 2021 and divested all stock holdings in Keyron in September 2021. He continues to provide scientific advice to Keyron for no remuneration. DAD receive research support from Eli Lilly and Company and participates on the Diabetes Advisory Board for Eli Lilly and Company and the steering committee for the tirzepatide CVOT. RJW, IP, KB, GJW, MZ and ICR are employees and shareholders of Eli Lilly and Company.
Figures
References
-
- National Center for Health Statistics (US). Health, United States, 2015: With Special Feature on Racial and Ethnic Health Disparities. Hyattsville (MD): National Center for Health Statistics (US); 2016. Report No.: 2016–1232. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

