Myeloid Deletion of Cdc42 Protects Liver From Hepatic Ischemia-Reperfusion Injury via Inhibiting Macrophage-Mediated Inflammation in Mice
- PMID: 38342302
- PMCID: PMC11047801
- DOI: 10.1016/j.jcmgh.2024.01.023
Myeloid Deletion of Cdc42 Protects Liver From Hepatic Ischemia-Reperfusion Injury via Inhibiting Macrophage-Mediated Inflammation in Mice
Abstract
Background & aims: Hepatic ischemia-reperfusion injury (HIRI) often occurs in liver surgery, such as partial hepatectomy and liver transplantation, in which myeloid macrophage-mediated inflammation plays a critical role. Cell division cycle 42 (Cdc42) regulates cell migration, cytoskeleton rearrangement, and cell polarity. In this study, we explore the role of myeloid Cdc42 in HIRI.
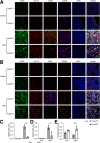
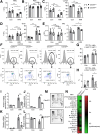
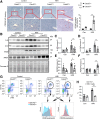
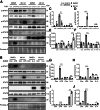
Methods: Mouse HIRI models were established with 1-hour ischemia followed by 12-hour reperfusion in myeloid Cdc42 knockout (Cdc42mye) and Cdc42flox mice. Myeloid-derived macrophages were traced with RosamTmG fluorescent reporter under LyzCre-mediated excision. The experiments for serum or hepatic enzymic activities, histologic and immunologic analysis, gene expressions, flow cytometry analysis, and cytokine antibody array were performed.
Results: Myeloid deletion of Cdc42 significantly alleviated hepatic damages with the reduction of hepatic necrosis and inflammation, and reserved hepatic functions following HIRI in mice. Myeloid Cdc42 deficiency suppressed the infiltration of myeloid macrophages, reduced the secretion of proinflammatory cytokines, restrained M1 polarization, and promoted M2 polarization of myeloid macrophages in livers. In addition, inactivation of Cdc42 promoted M2 polarization via suppressing the phosphorylation of STAT1 and promoting phosphorylation of STAT3 and STAT6 in myeloid macrophages. Furthermore, pretreatment with Cdc42 inhibitor, ML141, also protected mice from hepatic ischemia-reperfusion injury.
Conclusions: Inhibition or deletion of myeloid Cdc42 protects liver from HIRI via restraining the infiltration of myeloid macrophages, suppressing proinflammatory response, and promoting M2 polarization in macrophages.
Keywords: Cell Division Cycle 42 (Cdc42); Hepatic Ischemia-Reperfusion Injury; Macrophage; Polarization.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Figures





References
-
- Saidi R.F., Kenari S.K. Liver ischemia/reperfusion injury: an overview. J Invest Surg. 2014;27:366–379. - PubMed
-
- Hirao H., Nakamura K., Kupiec-Weglinski J.W. Liver ischaemia-reperfusion injury: a new understanding of the role of innate immunity. Nat Rev Gastroenterol Hepatol. 2022;19:239–256. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

