Targeting GRP75 with a Chlorpromazine Derivative Inhibits Endometrial Cancer Progression Through GRP75-IP3R-Ca2+-AMPK Axis
- PMID: 38342610
- PMCID: PMC11022737
- DOI: 10.1002/advs.202304203
Targeting GRP75 with a Chlorpromazine Derivative Inhibits Endometrial Cancer Progression Through GRP75-IP3R-Ca2+-AMPK Axis
Abstract
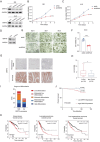
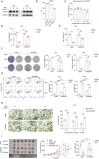
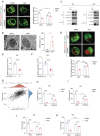
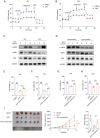
Tumors often overexpress glucose-regulated proteins, and agents that interfere with the production or activity of these proteins may represent novel cancer treatments. The chlorpromazine derivative JX57 exhibits promising effects against endometrial cancer with minimal extrapyramidal side effects; however, its mechanisms of action are currently unknown. Here, glucose-regulated protein 75 kD (GRP75) is identified as a direct target of JX57 using activity-based protein profiling and loss-of-function experiments. The findings show that GRP75 is necessary for the biological activity of JX57, as JX57 exhibits moderate anticancer properties in GRP75-deficient cancer cells, both in vitro and in vivo. High GRP75 expression is correlated with poor differentiation and poor survival in patients with endometrial cancer, whereas the knockdown of GRP75 can significantly suppress tumor growth. Mechanistically, the direct binding of JX57 to GRP75 impairs the structure of the mitochondria-associated endoplasmic reticulum membrane and disrupts the endoplasmic reticulum-mitochondrial calcium homeostasis, resulting in a mitochondrial energy crisis and AMP-activated protein kinase activation. Taken together, these findings highlight GRP75 as a potential prognostic biomarker and direct therapeutic target in endometrial cancer and suggest that the chlorpromazine derivative JX57 can potentially be a new therapeutic option for endometrial cancer.
Keywords: GRP75; MAM; chlorpromazine; endometrial cancer.
© 2024 The Authors. Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
IP3R-Grp75-VDAC1-MCU calcium regulation axis antagonists protect podocytes from apoptosis and decrease proteinuria in an Adriamycin nephropathy rat model.BMC Nephrol. 2018 Jun 15;19(1):140. doi: 10.1186/s12882-018-0940-3. BMC Nephrol. 2018. PMID: 29907098 Free PMC article.
-
Potential targets for the treatment of MI: GRP75-mediated Ca2+ transfer in MAM.Eur J Pharmacol. 2024 May 15;971:176530. doi: 10.1016/j.ejphar.2024.176530. Epub 2024 Mar 23. Eur J Pharmacol. 2024. PMID: 38527700
-
GRP75-faciliated Mitochondria-associated ER Membrane (MAM) Integrity controls Cisplatin-resistance in Ovarian Cancer Patients.Int J Biol Sci. 2022 Apr 11;18(7):2914-2931. doi: 10.7150/ijbs.71571. eCollection 2022. Int J Biol Sci. 2022. PMID: 35541901 Free PMC article.
-
Discovery of Novel Azaphenothiazine Derivatives to Suppress Endometrial Cancer by Targeting GRP75 to Impair Its Interaction with IP3R and Mitochondrial Ca2+ Homeostasis.J Med Chem. 2024 Aug 22;67(16):13829-13851. doi: 10.1021/acs.jmedchem.4c00638. Epub 2024 Jul 31. J Med Chem. 2024. PMID: 39082833
-
The ER-mitochondria interface, where Ca2+ and cell death meet.Cell Calcium. 2023 Jun;112:102743. doi: 10.1016/j.ceca.2023.102743. Epub 2023 Apr 25. Cell Calcium. 2023. PMID: 37126911 Review.
Cited by
-
A Small-Molecule Drug for the Self-Checking of Mitophagy.Angew Chem Int Ed Engl. 2025 Mar 3;64(10):e202421269. doi: 10.1002/anie.202421269. Epub 2025 Jan 22. Angew Chem Int Ed Engl. 2025. PMID: 39800659 Free PMC article.
-
Neuroscience of cancer: unraveling the complex interplay between the nervous system, the tumor and the tumor immune microenvironment.Mol Cancer. 2025 Jan 17;24(1):24. doi: 10.1186/s12943-024-02219-0. Mol Cancer. 2025. PMID: 39825376 Free PMC article. Review.
-
Repurposing of nervous system drugs for cancer treatment: recent advances, challenges, and future perspectives.Discov Oncol. 2025 Mar 26;16(1):396. doi: 10.1007/s12672-025-02067-4. Discov Oncol. 2025. PMID: 40133751 Free PMC article. Review.
-
The IP3R inhibitor desmethylxestospongin B reduces tumor cell migration, invasion and metastasis by impairing lysosome acidification and β1-integrin recycling.Biochim Biophys Acta Mol Basis Dis. 2025 Jan;1871(1):167557. doi: 10.1016/j.bbadis.2024.167557. Epub 2024 Oct 31. Biochim Biophys Acta Mol Basis Dis. 2025. PMID: 39486657 Free PMC article.
-
EphA5 Expression Predicts Better Survival Despite an Association with Proliferative Activity in Endometrial Cancer.J Clin Med. 2025 Jul 29;14(15):5360. doi: 10.3390/jcm14155360. J Clin Med. 2025. PMID: 40806980 Free PMC article.
References
-
- Sung H., Ferlay J., Siegel R. L., Laversanne M., Soerjomataram I., Jemal A., Bray F., CA Cancer J. Clin. 2021, 71, 209. - PubMed
-
- Son J., Carr C., Yao M., Radeva M., Priyadarshini A., Marquard J., Michener C. M., AlHilli M., Int. J. Gynecol. Cancer : Off. J. Int. Gynecol. Cancer Soc. 2020, 30, 631. - PubMed
-
- Bovicelli A., D'Andrilli G., Giordano A., De Iaco P., J. Cell. Physiol. 2013, 228, 1154. - PubMed
MeSH terms
Substances
Grants and funding
- 22XD1403500/Program of Shanghai Academic Research Leader
- YDZX20223100003006/Central Guidance on Local Science and Technology Development Fund of Shanghai Province
- shslczdzk06302/Shanghai Municipal Key Clinical Specialty
- 20550760600/"Science and Technology Innovation Action Plan" International Science and Technology Cooperation
- YG2022ZD/Shanghai Jiao Tong University Medicine-Engineering Fund
LinkOut - more resources
Full Text Sources
