Analysis of the transplacental transmission of SARS CoV-2 virus and antibody transfer according to the gestational age at maternal infection
- PMID: 38342940
- PMCID: PMC10859378
- DOI: 10.1038/s41598-024-53580-5
Analysis of the transplacental transmission of SARS CoV-2 virus and antibody transfer according to the gestational age at maternal infection
Abstract
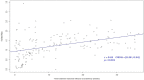
To quantify transplacental transmission of SARS-CoV-2 virus and antibody transfer in pregnant women and their newborns according to the gestational age at maternal infection. A prospective observational multicenter study including pregnant women with a positive RT-PCR or a positive serology for SARS-CoV-2 and compatible symptoms, from April to December 2020, in 11 French maternities. The study was designed to obtain a systematic collection of mother-infant dyad's samples at birth. SARS-CoV-2 viral load was measured by RT-PCR. IgG and IgM antibodies against the SARS-CoV-2 spike protein were measured by enzyme-linked immunosorbent assay. Antibody concentrations and transplacental transfer ratios were analyzed according to the gestational age at maternal infection. The primary outcome was the rate of SARS CoV-2 materno-fetal transmission at birth. The secondary outcome was the quantification of materno-fetal antibody transfer. Maternal and neonatal outcomes at birth were additionally assessed. Among 165 dyads enrolled, one congenital infection was confirmed {n = 1 (0.63%) IC95% [0.02%; 3.48%]}. The average placental IgG antibody transfer ratio was 1.27 (IC 95% [0.69-2.89]). The transfer ratio increased with increasing time between the onset of maternal infection and delivery (P Value = 0.0001). Maternal and neonatal outcomes were reassuring. We confirmed the very low rate of SARS-CoV-2 transplacental transmission (< 1%). Maternal antibody transfer to the fetus was more efficient when the infection occurred during the first and second trimester of pregnancy.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous