Simulation-based evaluation of personalized dosing approaches for anti-FGFR/KLB bispecific antibody fazpilodemab
- PMID: 38343040
- PMCID: PMC11015072
- DOI: 10.1002/psp4.13111
Simulation-based evaluation of personalized dosing approaches for anti-FGFR/KLB bispecific antibody fazpilodemab
Abstract
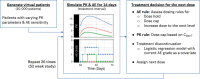
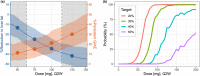
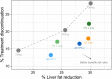
Personalized dosing approaches play important roles in clinical practices to improve benefit: risk profiles. Whereas this is also important for drug development, especially in the context of drugs with narrow therapeutic windows, such approaches have not been fully evaluated during clinical development. Fazpilodemab (BFKB8488A) is an agonistic bispecific antibody which was being developed for the treatment of nonalcoholic steatohepatitis. The objective of this study was to characterize the exposure-response relationships of fazpilodemab with the purpose of guiding dose selection for a phase II study, as well as to evaluate various personalized dosing strategies to optimize the treatment benefit. Fazpilodemab exhibited clear exposure-response relationships for a pharmacodynamic (PD) biomarker and gastrointestinal adverse events (GIAEs), such as nausea and vomiting. Static exposure-response analysis, as well as longitudinal adverse event (AE) analysis using discrete-time Markov model, were performed to characterize the observations. Clinical trial simulations were performed based on the developed exposure-response models to evaluate probability of achieving target PD response and the frequency of GIAEs to inform phase II dose selection. Dynamic simulation of personalized dosing strategies demonstrated that the AE-based personalized dosing is the most effective approach for optimizing the benefit-risk profiles. The approach presented here can be a useful framework for quantifying the benefit of personalized dosing for drugs with narrow therapeutic windows.
© 2024 Genentech, Inc. CPT: Pharmacometrics & Systems Pharmacology published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
All authors are current or former employees of Genentech, Inc. (a member of the Roche group) and own or owned stock in F. Hoffman‐La Roche.
Figures
References
-
- Darwich AS, Polasek TM, Aronson JK, et al. Model‐informed precision dosing: background, requirements, validation, implementation, and forward trajectory of individualizing drug therapy. Annu Rev Pharmacol Toxicol. 2020;61:1‐21. - PubMed
-
- Barrett JS, Barrett RF, Vinks AA. Status toward the implementation of precision dosing in children. J Clin Pharmacol. 2021;61:S36‐S51. - PubMed
-
- Peck R. Precision medicine is not just genomics: the right dose for every patient. Annu Rev Pharmacol Toxicol. 2017;58:1‐18. - PubMed
-
- Vinks AA, Peck RW, Neely M, Mould DR. Development and implementation of electronic health record–integrated model‐informed clinical decision support tools for the precision dosing of drugs. Clin Pharmacol Ther. 2020;107:129‐135. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

