Appetite and dietary intake endpoints in cancer cachexia clinical trials: Systematic Review 2 of the cachexia endpoints series
- PMID: 38343065
- PMCID: PMC10995275
- DOI: 10.1002/jcsm.13434
Appetite and dietary intake endpoints in cancer cachexia clinical trials: Systematic Review 2 of the cachexia endpoints series
Abstract
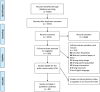
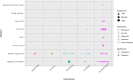
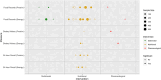
There is no consensus on the optimal endpoint(s) in cancer cachexia trials. Endpoint variation is an obstacle when comparing interventions and their clinical value. The aim of this systematic review was to summarize and evaluate endpoints used to assess appetite and dietary intake in cancer cachexia clinical trials. A search for studies published from 1 January 1990 until 2 June 2021 was conducted using MEDLINE, Embase and Cochrane Central Register of Controlled Trials. Eligible studies examined cancer cachexia treatment versus a comparator in adults with assessments of appetite and/or dietary intake as study endpoints, a sample size ≥40 and an intervention lasting ≥14 days. Reporting was in line with PRISMA guidance, and a protocol was published in PROSPERO (2022 CRD42022276710). This review is part of a series of systematic reviews examining cachexia endpoints. Of the 5975 articles identified, 116 were eligible for the wider review series and 80 specifically examined endpoints of appetite (65 studies) and/or dietary intake (21 studies). Six trials assessed both appetite and dietary intake. Appetite was the primary outcome in 15 trials and dietary intake in 7 trials. Median sample size was 101 patients (range 40-628). Forty-nine studies included multiple primary tumour sites, while 31 studies involved single primary tumour sites (15 gastrointestinal, 7 lung, 7 head and neck and 2 female reproductive organs). The most frequently reported appetite endpoints were visual analogue scale (VAS) and numerical rating scale (NRS) (40%). The appetite item from the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ) C30/C15 PAL (38%) and the appetite question from North Central Cancer Treatment Group anorexia questionnaire (17%) were also frequently applied. Of the studies that assessed dietary intake, 13 (62%) used food records (prospective registrations) and 10 (48%) used retrospective methods (24-h recall or dietary history). For VAS/NRS, a mean change of 1.3 corresponded to Hedge's g of 0.5 and can be considered a moderate change. For food records, a mean change of 231 kcal/day or 11 g of protein/day corresponded to a moderate change. Choice of endpoint in cachexia trials will depend on factors pertinent to the trial to be conducted. Nevertheless, from trials assessed and available literature, NRS or EORTC QLQ C30/C15 PAL seems suitable for appetite assessments. Appetite and dietary intake endpoints are rarely used as primary outcomes in cancer cachexia. Dietary intake assessments were used mainly to monitor compliance and are not validated in cachexia populations. Given the importance to cachexia studies, dietary intake endpoints must be validated before they are used as endpoints in clinical trials.
Keywords: appetite; cachexia; cancer; dietary intake; endpoints; outcomes; trials.
© 2024 The Authors. Journal of Cachexia, Sarcopenia and Muscle published by Wiley Periodicals LLC.
Conflict of interest statement
MF has received personal fees from Pfizer. MJH has received funding from CRUK, NIH National Cancer Institute, IASLC International Lung Cancer Foundation, Lung Cancer Research Foundation, Rosetrees Trust, UKI NETS and NIHR. MJH has consulted for, and is a member of, the Achilles Therapeutics Scientific Advisory Board and Steering Committee; has received speaker honoraria from Pfizer, Astex Pharmaceuticals, Oslo Cancer Cluster and Bristol Myers Squibb; and is a co‐inventor on a European patent application relating to methods to detect lung cancer (PCT/US2017/028013). RJES has received personal fees for consultancy from Artelo, Actimed, Faraday and Helsinn. BJAL has received personal fees for consultancy from Artelo, Actimed, Faraday, Kyowa Kirin and Toray. The remaining authors have not reported any conflicts of interest.
Figures
References
-
- Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12:489–495. - PubMed
-
- Stratton RJ. Elucidating effective ways to identify and treat malnutrition. Proc Nutr Soc 2005;64:305–311. - PubMed
-
- Argiles JM, Lopez‐Soriano FJ, Stemmler B, Busquets S. Cancer‐associated cachexia—understanding the tumour macroenvironment and microenvironment to improve management. Nat Rev Clin Oncol 2023;20:250–264. - PubMed
-
- Barajas Galindo DE, Vidal‐Casariego A, Calleja‐Fernández A, Hernández‐Moreno A, Pintor de la Maza B, Pedraza‐Lorenzo M, et al. Appetite disorders in cancer patients: impact on nutritional status and quality of life. Appetite 2017;114:23–27. - PubMed
-
- Baracos VE, Martin L, Korc M, Guttridge DC, Fearon KCH. Cancer‐associated cachexia. Nat Rev Dis Primers 2018;4:17105. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

