Effect of Ligand Substituents on Spectroscopic and Catalytic Properties of Water-Compatible Cp*Ir-(pyridinylmethyl)sulfonamide-Based Transfer Hydrogenation Catalysts
- PMID: 38343274
- PMCID: PMC10900292
- DOI: 10.1021/acs.inorgchem.3c04040
Effect of Ligand Substituents on Spectroscopic and Catalytic Properties of Water-Compatible Cp*Ir-(pyridinylmethyl)sulfonamide-Based Transfer Hydrogenation Catalysts
Abstract
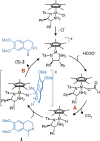
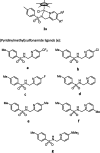
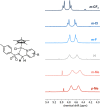
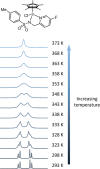
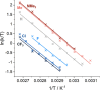
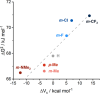
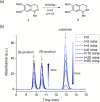
Transition-metal-based hydrogenation catalysts have applications ranging from high-value chemical synthesis to medicinal chemistry. A series of (pyridinylmethyl)sulfonamide ligands substituted with electron-withdrawing and -donating groups were synthesized to study the influence of the electronic contribution of the bidentate ligand in Cp*Ir piano-stool complexes. A variable-temperature NMR investigation revealed a strong correlation between the electron-donating ability of the substituent and the rate of stereoinversion of the complexes. This correlation was partially reflected in the catalytic activity of the corresponding catalysts. Complexes with electron-withdrawing substituents followed the trend observed in the variable-temperature NMR study, thereby confirming the rate-determining step to be donation of the hydride ligand. Strongly electron-donating groups, on the other hand, caused a change in the rate-determining step in the formation of the iridium-hydride species. These results demonstrate that the activity of these catalysts can be tuned systematically via changes in the electronic contribution of the bidentate (pyridinylmethyl)sulfonamide ligands.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




Similar articles
-
Efficient Iridium Catalysts for Formic Acid Dehydrogenation: Investigating the Electronic Effect on the Elementary β-Hydride Elimination and Hydrogen Formation Steps.Inorg Chem. 2021 Mar 1;60(5):3410-3417. doi: 10.1021/acs.inorgchem.0c03815. Epub 2021 Feb 9. Inorg Chem. 2021. PMID: 33560831
-
Iridium Complexes with Bidentate Pyridinylidene/N-Amidate Ligands for Transfer Hydrogenation Catalysis.Inorg Chem. 2024 Aug 26;63(34):15724-15734. doi: 10.1021/acs.inorgchem.4c01630. Epub 2024 Aug 8. Inorg Chem. 2024. PMID: 39115421
-
Chiral-at-Metal: Iridium(III) Tetrazole Complexes With Proton-Responsive P-OH Groups for CO2 Hydrogenation.Front Chem. 2020 Nov 13;8:591353. doi: 10.3389/fchem.2020.591353. eCollection 2020. Front Chem. 2020. PMID: 33304883 Free PMC article.
-
Tuning the Electronic Properties of Azophosphines as Ligands and Their Application in Base-Free Transfer Hydrogenation Catalysis.Organometallics. 2024 Sep 6;43(20):2674-2685. doi: 10.1021/acs.organomet.4c00302. eCollection 2024 Oct 28. Organometallics. 2024. PMID: 39483130 Free PMC article.
-
Recent advances in osmium-catalyzed hydrogenation and dehydrogenation reactions.Acc Chem Res. 2015 Feb 17;48(2):363-79. doi: 10.1021/ar5003818. Epub 2015 Feb 4. Acc Chem Res. 2015. PMID: 25650714 Review.
References
-
- James B. R. Synthesis of Chiral Amines Catalyzed Homogeneously by Metal Complexes. Catal. Today 1997, 37, 209–221. 10.1016/S0920-5861(97)00011-4. - DOI
-
- Nugent T. C.; El-Shazly M. Chiral Amine Synthesis - Recent Developments and Trends for Enamide Reduction, Reductive Amination, and Imine Reduction. Adv. Synth. Catal. 2010, 352, 753–819. 10.1002/adsc.200900719. - DOI
-
- Hashiguchi S.; Fujii A.; Takehara J.; Ikariya T.; Noyori R. Asymmetric Transfer Hydrogenation of Aromatic Ketones Catalyzed by Chiral Ruthenium (II) Complexes. J. Am. Chem. Soc. 1995, 117, 7562–7563. 10.1021/ja00133a037. - DOI
-
- Noyori R.; Hashiguchi S. Asymmetric Transfer Hydrogenation Catalyzed by Chiral Ruthenium Complexes. Acc. Chem. Res. 1997, 30 (2), 97–102. 10.1021/ar9502341. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

