Clinical Characteristics and Outcomes of Polyarteritis Nodosa: An International Study
- PMID: 38343337
- PMCID: PMC11213674
- DOI: 10.1002/art.42817
Clinical Characteristics and Outcomes of Polyarteritis Nodosa: An International Study
Abstract
Objective: We describe the demographics, clinical features, disease course, and survival of polyarteritis nodosa (PAN) through an international collaboration (GLOBAL-PAN).
Methods: Patients with PAN were recruited between 1990 and 2020 from observational cohorts of nine countries across Europe, Japan, and North America. Eligibility was retrospectively defined using the European Medicines Agency classification algorithm. Patients with PAN related to hepatitis B virus (n = 12) and two monogenic diseases mimicking PAN, deficiency of adenosine deaminase 2 enzyme (n = 16) or familial Mediterranean fever (n = 11), were excluded. Data regarding organ involvement, relapse, disease-related damage, and survival were analyzed.
Results: Three hundred fifty-eight patients (female:male ratio 174:184), including those with systemic PAN (sPAN, n = 282) and cutaneous PAN (n = 76), were included. Twenty-five were pediatric onset. Mean ± SD age at diagnosis was 44.3 ± 18.1 years. Constitutional symptoms (71.5%), cutaneous involvement (70.5%), musculoskeletal findings (69.1%), and neurologic features (48.0%) were common manifestations. Among patients with sPAN, gastrointestinal involvement and proteinuria over 400 mg/day were reported in 52.2% and 11.2%, respectively. During a median (interquartile range) 59.6 (99.5) months of follow-up, relapse occurred in 48.5% of patients. One, 5- and 10-year survival rates for sPAN were 97.1%, 94.0%, and 89.0%, respectively. Predictors of death for sPAN included age ≥65 years at diagnosis, serum creatinine at diagnosis >140 μmol/L, gastrointestinal manifestations, and central nervous system (CNS) involvement.
Conclusion: The spectrum of PAN remains a complex, multifaceted disease. Relapse is common. Age ≥65 years and serum creatinine >140 μmol/L at diagnosis, as well as gastrointestinal and CNS involvement, are independent predictors of death in sPAN.
© 2024 American College of Rheumatology.
Conflict of interest statement
COI: OK declares Research grants from AbbVie, Novartis, Viela-Bio, TR-Pharma, Consulting fees from AbbVie, Abdi İbrahim, Celltrion, Novartis, Pfizer, Sandoz, UCB-Pharma
COI: ECB declares no COI.
COI: GA declares no COI.
COI:
COI:
COI: CP declares grants from Chemocentryx, Otsuka, Pfizer, GSK, TEVA, consulting fees from Otsuka, AstraZeneca, Pfizer, Roche, honoraria for lectures, presentations, speaker’s bureaus, manuscript writing or educational events from Otsuka, AstraZeneca, GSK.
COI: EMN declares no COI.
COI: SM declares no COI.
COI: YA declares no COI.
COI: FA declares, consulting fees from CSL VIFOR, AstraZeneca, Boehringer and honoraria from CSL VIFOR, AstraZeneca, Otsuka, Boehringer, Alexion, Novartis and Payment for expert testimony from CSL VIFOR, Boehringer and Support for attending meetings and/or travel from CSL VIFOR.
COI: FA declares no COI.
COI: DC declares no COI.
COI: LD declares no COI.
COI: HD declares no COI.
COI: NK declares grants for clinical trial from BMS, AbbVie, Sanofi and consulting fees from Roche and honoraria from Otsuka, GSK and Mallinckrodt.
COI: CK declares no COI
COI: CL declares funding to institution from National Institutes of Health.
COI: CM declares no COI.
COI: PM declares consulting fees from Hi-Bio, ChemoCentryx and honorarium for lecture from Genentech.
COI: LM declares consulting fees and honoraria from GSK, Astra Zeneca and support for attending meetings and/or travel from Vifor Pharma and participation fees on a Data Safety Monitoring Board or Advisory Board from GSK, Astra Zeneca.
COI: RP declares no COI.
COI: PS declares no COI.
COI: KW declares grants from Eli Lilly, Kliniksa, BMS and consulting fees from Amgen and Sanofi.
COI: AH declares no COI.
COI: AHA declares no COI.
COI: SF declares consulting fees from Asahi Kasei Pharma, honoraria for lectures from Asahi Kasei Pharma, Chugai Pharmaceutical, Daiichi Sankyo, Eisai, KISSEI Pharmaceutical, Otsuka Pharmaceutical
COI: GE declares honoraria from GSK, AstraZeneca, Vifor, SOBI, Novartis, Boehringer.
COI: SO declares no COI.
COI: DJ declares grants to the institution from GSK, Roche and consulting fees from Astra-Zeneca, GSK, Novartis, Roche, Takeda, CSL Vifor and honoraria from Amgen, GSK, CSL-Vifor, participation fees on a Data Safety Monitoring Board or Advisory Board from Chinook, GSK, Boehringer and Leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid for European Vasculitis Society and stock or stock options from Aurinia, Alentis.
COI: PM declares grants to the institution from AbbVie, Amgen, AstraZeneca, Boeringher-Ingelheim, Bristol-Myers Squibb, Eicos, Electra, Forbius, Genentech/Roche, GlaxoSmithKline, InflaRx, Neutrolis, Sanofi, Takeda.. Royalties/licences from Upto Date and consultin fees from AbbVie, Amgen, ArGenx, AstraZeneca, Boeringher-Ingelheim, Bristol-Myers Squibb, Cabaletta, CSL Behring, GlaxoSmithKline, HiBio, InflaRx, Janssen, Jubilant, Kyverna, MiroBio, Novartis, NS Pharma, Q32, Regeneron, Sparrow, Takeda, Vistera.
Figures
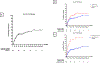

References
-
- Jennette JC, Falk RJ, Andrassy K, Bacon PA, Churg J, Gross WL, et al. Nomenclature of systemic vasculitides. Proposal of an international consensus conference. Arthritis Rheum. 1994;37(2):187–92. - PubMed
-
- Mahr A, Guillevin L, Poissonnet M, Ayme S. Prevalences of polyarteritis nodosa, microscopic polyangiitis, Wegener’s granulomatosis, and Churg-Strauss syndrome in a French urban multiethnic population in 2000: a capture-recapture estimate. Arthritis Rheum. 2004;51(1):92–9. - PubMed
-
- Lightfoot RW Jr., Michel BA, Bloch DA, Hunder GG, Zvaifler NJ, McShane DJ, et al. The American College of Rheumatology 1990 criteria for the classification of polyarteritis nodosa. Arthritis Rheum. 1990;33(8):1088–93. - PubMed
-
- Scott DGI, Watts RA. Epidemiology and clinical features of systemic vasculitis. Clin Exp Nephrol. 2013;17(5):607–10. - PubMed
Publication types
MeSH terms
Grants and funding
- National Institute for Health Research Cambridge Biomedical Research Centre
- ZIA AR041204/ImNIH/Intramural NIH HHS/United States
- U54 RR019497/RR/NCRR NIH HHS/United States
- U54-AR-057319/National Center for Advancing Translational Science, the National Institute of Arthritis and Musculoskeletal and Skin Diseases
- U54-RR-019497/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials

