Ostial right coronary artery lesion morphology and outcomes after treatment with drug-eluting stents
- PMID: 38343369
- PMCID: PMC10836391
- DOI: 10.4244/EIJ-D-23-00406
Ostial right coronary artery lesion morphology and outcomes after treatment with drug-eluting stents
Abstract
Background: Outcomes after percutaneous coronary intervention (PCI) for de novo ostial right coronary artery (RCA) lesions are poor.
Aims: We used intravascular ultrasound (IVUS) to clarify the morphological patterns of de novo ostial RCA lesions and their associated clinical outcome.
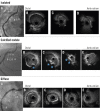
Methods: Among 5,102 RCA IVUS studies, 170 de novo ostial RCA stenoses (within 3 mm from the aorto-ostium) were identified. These were classified as 1) isolated ostial lesions (no disease extending beyond 10 mm from the ostium and without a calcified nodule [CN]); 2) ostial CN, typically with diffuse disease (disease extending beyond 10 mm); and 3) ostial lesions with diffuse disease but without a CN. The primary outcome was target lesion failure (TLF: cardiac death, target vessel myocardial infarction, definite stent thrombosis, and ischaemia-driven target lesion revascularisation).
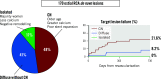
Results: The prevalence of an isolated ostial lesion was 11.8% (n=20), 47.6% (n=81) were ostial CN, and 40.6% (n=69) were ostial lesions with diffuse disease. Compared to ostial lesions with diffuse disease, isolated lesions were more common in women (75.0% vs 42.0%; p=0.01), and CN were associated with older age (median [first, third quartile] 76 [70, 83] vs 69 [63, 81] years old; p=0.002). The Kaplan-Meier rate of TLF at 2 years was significantly higher in patients with CN (21.6%) compared to diffuse lesions (8.2%) (p=0.04), and patients with isolated lesions had no events. A multivariable Cox proportional hazard model revealed that CN were significantly associated with TLF (hazard ratio 6.63, 95% confidence interval: 1.28-34.3; p=0.02).
Conclusions: Ostial RCA lesions have specific morphologies - detectable by IVUS - that may be associated with long-term clinical outcomes.
Conflict of interest statement
M. Matsumura is a consultant for Terumo Corporation and Boston Scientific. K.N. Fall is a consultant for Infraredx and Boston Scientific. M. Prasad is a consultant for and is on the speakers bureau of Conavi Medical, Neovasc, Abbott, Cardinol, Chiesi, and Boehringer Ingelheim. V.G. Ng receives speaker honoraria from Edwards Lifesciences and Medtronic. T.M. Nazif is a consultant for and receives speaker honoraria from Medtronic and Boston Scientific. S.A. Parikh has done research for Abbott, Boston Scientific, Medtronic, Philips, Cordis, and Jannsen; is a consultant for Inari, Penumbra, Terumo, and Canon; and holds equity in Efemoral Medical, Advanced NanoTherapies, and Encompass Vascular. D. Karmpaliotis received speaker honoraria from Boston Scientific and Abbott; and holds equity in Saranas, SoundBite Medical Solutions, and Traverse Vascular. M.B. Leon received institutional clinical research grants from Abbott, Boston Scientific, and Medtronic. M. McEntegart received speaker honoraria from Boston Scientific, Abbott, Shockwave Medical, Teleflex, and Biosensors. J.W. Moses has equity in Orchestra BioMed and Xenter. A.J. Kirtane received institutional funding from Medtronic, Boston Scientific, Abbott, Amgen, CathWorks, CSI, Philips, ReCor Medical, Neurotronics, Biotronik, Chiesi, Bolt Medical, Magenta Medical, Canon, SoniVie, and Shockwave Medical (institutional funding includes fees paid to Columbia University and/or Cardiovascular Research Foundation for consulting and/or speaking engagements in which A.J. Kirtane controlled the content); is a consultant for IMDS; received travel expenses/meals from Amgen, Medtronic, Biotronik, Boston Scientific, Abbott, CathWorks, Edwards Lifesciences, CSI, Novartis, Philips, Abiomed, ReCor Medical, Chiesi, Zoll, Shockwave Medical, and Regeneron. G.S. Mintz received speaker honoraria from Boston Scientific, Philips, SpectraWAVE, and Gentuity. A. Maehara is a consultant for Boston Scientific, Shockwave Medical, and SpectraWAVE. The other authors have no conflicts of interest to declare.
Figures

References
-
- Piccolo R, Bonaa KH, Efthimiou O, Varenne O, Baldo A, Urban P, Kaiser C, Remkes W, Raber L, de Belder, van ‘t, Stankovic G, Lemos PA, Wilsgaard T, Reifart J, Rodriguez AE, Ribeiro EE, Serruys PWJC, Abizaid A, Sabate M, Byrne RA, de la, Wijns W, Juni P, Windecker S, Valgimigli M Coronary Stent Trialists’ Collaboration. Drug-eluting or bare-metal stents for percutaneous coronary intervention: a systematic review and individual patient data meta-analysis of randomised clinical trials. Lancet. 2019;393:2503–10. - PubMed
-
- Lam MK, Sen H, Tandjung K, Lowik MM, Basalus MW, Mewes JC, Stoel MG, van Houwelingen, Linssen GC, Ijzerman MJ, Doggen CJ, von Birgelen. Clinical outcome of patients with implantation of second-generation drug-eluting stents in the right coronary ostium: insights from 2-year follow-up of the TWENTE trial. Catheter Cardiovasc Interv. 2015;85:524–31. - PubMed
-
- Kan J, Ge Z, Zhang JJ, Liu ZZ, Tian NL, Ye F, Li SJ, Qian XS, Yang S, Chen MX, Rab T, Chen SL. Incidence and Clinical Outcomes of Stent Fractures on the Basis of 6,555 Patients and 16,482 Drug-Eluting Stents From 4 Centers. JACC Cardiovasc Interv. 2016;9:1115–23. - PubMed
-
- Topol EJ, Ellis SG, Fishman J, Leimgruber P, Myler RK, Stertzer SH, O’Neill WW, Douglas JS, Roubin GS, King SB. Multicenter study of percutaneous transluminal angioplasty for right coronary artery ostial stenosis. J Am Coll Cardiol. 1987;9:1214–8. - PubMed
-
- Darabian S, Amirzadegan AR, Sadeghian H, Sadeghian S, Abbasi A, Raeesi M. Ostial lesions of left main and right coronary arteries: demographic and angiographic features. Angiology. 2008;59:682–7. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

