Murine IRF8 Mutation Offers New Insight into Osteoclast and Root Resorption
- PMID: 38343385
- PMCID: PMC10985390
- DOI: 10.1177/00220345231222173
Murine IRF8 Mutation Offers New Insight into Osteoclast and Root Resorption
Abstract
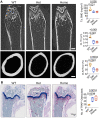
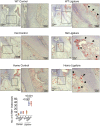
Interferon regulatory factor 8 (IRF8), a transcription factor expressed in immune cells, functions as a negative regulator of osteoclasts and helps maintain dental and skeletal homeostasis. Previously, we reported that a novel mutation in the IRF8 gene increases susceptibility to multiple idiopathic cervical root resorption (MICRR), a form of tooth root resorption mediated by increased osteoclast activity. The IRF8 G388S variant in the highly conserved C-terminal motif is predicted to alter the protein structure, likely impairing IRF8 function. To investigate the molecular basis of MICRR and IRF8 function in osteoclastogenesis, we generated Irf8 knock-in (KI) mice using CRISPR/Cas9 technique modeling the human IRF8G388S mutation. The heterozygous (Het) and homozygous (Homo) Irf8 KI mice showed no gross morphological defects, and the development of hematopoietic cells was unaffected and similar to wild-type (WT) mice. The Irf8 KI Het and Homo mice showed no difference in macrophage gene signatures important for antimicrobial defenses and inflammatory cytokine production. Consistent with the phenotype observed in MICRR patients, Irf8 KI Het and Homo mice demonstrated significantly increased osteoclast formation and resorption activity in vivo and in vitro when compared to WT mice. The oral ligature-inserted Het and Homo mice displayed significantly increased root resorption and osteoclast-mediated alveolar bone loss compared to WT mice. The increased osteoclastogenesis noted in KI mice is due to the inability of IRF8G388S mutation to inhibit NFATc1-dependent transcriptional activation and downstream osteoclast specific transcripts, as well as its impact on autophagy-related pathways of osteoclast differentiation. This translational study delineates the IRF8 domain important for osteoclast function and provides novel insights into the IRF8 mutation associated with MICRR. IRF8G388S mutation mainly affects osteoclastogenesis while sparing immune cell development and function. These insights extend beyond oral health and significantly advance our understanding of skeletal disorders mediated by increased osteoclast activity and IRF8's role in osteoclastogenesis.
Keywords: NFATC Transcription Factors; dental biology; genetic animal models; genetic mutation; human association studies; osteoclasts.
Conflict of interest statement
Declaration of Conflicting InterestsThe authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures



References
-
- Bigley V, Maisuria S, Cytlak U, Jardine L, Care MA, Green K, Gunawan M, Milne P, Dickinson R, Wiscombe S, et al. 2017. Biallelic interferon regulatory factor 8 mutation: a complex immunodeficiency syndrome with dendritic cell deficiency, monocytopenia, and immune dysregulation. J Allergy Clin Immunol. 141(6):2234–2248. - PMC - PubMed
-
- Darcey J, Qualtrough A. 2013. Resorption: part 1. Pathology, classification and aetiology. Br Dent J. 214(9):439–451. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

