Use of video-electroencephalography as a first-line examination in veterinary neurology: development and standardization of electroencephalography in unsedated dogs and cats
- PMID: 38343449
- PMCID: PMC10853351
- DOI: 10.3389/fvets.2024.1326165
Use of video-electroencephalography as a first-line examination in veterinary neurology: development and standardization of electroencephalography in unsedated dogs and cats
Abstract
Objective: To assess the feasibility and validate the use of video-electroencephalography (EEG) in conscious dogs and cats and to propose guidelines of routine EEG in veterinary clinical practice.
Design: Prospective clinical study.
Data: One hundred and fifty EEG recordings were carried out to validate the clinical adding-value, reproducibility, and guidelines on 140 owned animals. One hundred and one EEGs were performed on dogs and 49 on cats.
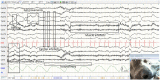
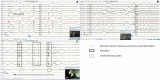
Procedures: We compared recordings performed with 8 EEG unwired stud Ag/AgCl electrodes held by elastic straps and 8 EEG wired cup Ag electrodes held by a tailor-made manufactured headset combined with a wired video-EEG device. Electrodes placement was determined according to previously published animal EEG protocols. Physiological sensors, such as electrocardiography, electromyography, and respiratory sensors were added. Stimulation protocols were tested. Quality and interpretability were evaluated.
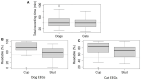
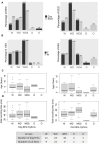
Results: Headsets and recording procedures appeared suitable for all skull shapes and sizes. Video-EEG recordings were successfully performed without tranquilization or anesthesia except for 9 animals. Median EEG recordings time was 40 min. Impedance remained below 20 kΩ in 99% of dog EEGs and 98% of cat EEGs. Isosynchrony was reported in 6% of the channels. Seventy-five percent of dog EEGs and 83% of cat EEGs were readable for more than 50% (to 100%) of their duration. Successful discrimination of vigilance states from rhythm analysis (wakefulness, drowsiness, and sleepiness) was possible in 99% of dog EEGs and 91% of cat EEGs. Photic driving responses during photic stimulations were observed in 11% of dog EEGs and 85% of cat EEGs. Electroencephalography recordings were directly informative in 32% of the examinations: in 25% EEG abnormalities were associated with clinical signs and 7% concerned EEG abnormalities without clinical symptoms during recording. Thirteen percent of dogs subjected to photic stimulation exhibited epileptic anomalies. Among 9 EEGs with other history-based stimulations, three displayed epileptic graphoelements.
Conclusions: We have developed a standardized unanesthetized video-EEG procedure easily performed and reproducible in dogs and cats. Qualitative and quantitative technical and medical criteria were evaluated and were in accordance with human EEG recommendations. Moreover, we have demonstrated its relevance and accuracy for diagnostic purposes, providing further arguments for the use of EEG as a first-line neurological functional exploration test.
Keywords: EEG; canine; encephalopathy; epilepsy; feline; gel electrode; video-EEG.
Copyright © 2024 Lyon, Pochat, Blot, Troupel, Van Caenegem, Besnard and Escriou.
Conflict of interest statement
EL was employed by AMSET MEDICAL. EL is the patent applicant No. FR3101533. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
LinkOut - more resources
Full Text Sources
Miscellaneous

