Increased circulating regulatory T cells and decreased follicular T helper cells are associated with colorectal carcinogenesis
- PMID: 38343544
- PMCID: PMC10853383
- DOI: 10.3389/fimmu.2024.1287632
Increased circulating regulatory T cells and decreased follicular T helper cells are associated with colorectal carcinogenesis
Abstract
Objective: Colorectal cancer (CRC) is the third most prevalent cancer worldwide and is associated with high morbidity and mortality rates. Colorectal carcinogenesis occurs via the conventional adenoma-to-carcinoma and serrated pathways. Conventional T helper (Th) and innate lymphoid cells (ILCs) play vital roles in maintaining intestinal homeostasis. However, the contribution of these two major lymphoid cell populations and their associated cytokines to CRC development is unclear. Therefore, we aimed to analyze peripheral lymphocyte profiles during colorectal carcinogenesis.
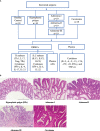
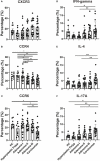
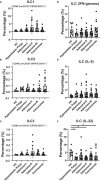
Methods: We collected 86 blood samples concurrently, and pathologists confirmed the presence of various pathological conditions (i.e., HPs, adenoma, and carcinoma) using hematoxylin and eosin staining. Ten healthy donors were recruited as healthy controls (HCs) from the physical examination center. We performed flow cytometry on peripheral blood mononuclear cells collected from patients with various pathological conditions and the HCs, and cytokines (interleukin-2, interleukin-4, interleukin-5, interleukin-13, interleukin-17A, interleukin-17F, interleukin-22, interferon-γ, and tumor necrosis factor-α) were quantified. We also analyzed the published single-cell RNA sequence data derived from tissue samples from different stages of colorectal carcinogenesis.
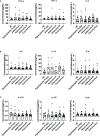
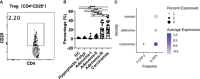
Results: The cytokine response in peripheral CD4+ T cells was upregulated during the carcinoma process. The frequency of peripheral regulatory T cells (Tregs) increased in the adenoma and carcinoma stages. While the T follicular helper (Tfh) cell proportion was downregulated in the adenoma and carcinoma processes. Thus, Th cell subsets, especially Tregs and Tfh cells, were involved in colonic diseases. Moreover, the immunological profile characteristics in the HPs were clarified.
Conclusion: We comprehensively analyzed circulating ILCs and adaptive T-cell lymphocyte subtypes in colorectal carcinoma progression. Our results show the immunological profile characteristics and support the involvement of Th subsets, especially Treg and Tfh cell populations, in colonic diseases. These findings significantly enhance our understanding of the immune mechanisms underlying CRC and its precancerous lesions. Further investigation of the Treg and Tfh cells' function in colorectal disease development will provide potential therapeutic targets for monitoring and preventing CRC development.
Keywords: T helper cell; adenoma; colorectal cancer; hyperplastic polyps; innate lymphoid cells.
Copyright © 2024 Meng, Zhao, Xu, Wang, Li, Cui, Fu and Ding.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Comment on
-
Differential pre-malignant programs and microenvironment chart distinct paths to malignancy in human colorectal polyps.Cell. 2021 Dec 22;184(26):6262-6280.e26. doi: 10.1016/j.cell.2021.11.031. Epub 2021 Dec 14. Cell. 2021. PMID: 34910928 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

