Irreversible inhibition of estrogen receptor α signaling and the emergence of hormonal resistance in MCF7 breast cancer cells induced by DNA damage agents
- PMID: 38343657
- PMCID: PMC10853760
- DOI: 10.3892/br.2024.1727
Irreversible inhibition of estrogen receptor α signaling and the emergence of hormonal resistance in MCF7 breast cancer cells induced by DNA damage agents
Abstract
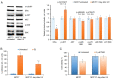
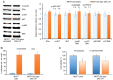
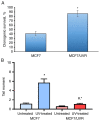
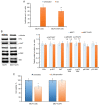
Combining chemotherapy and hormone therapy is a prevalent approach in breast cancer treatment. While the cytotoxic impact of numerous chemotherapy drugs stems from DNA damage, the exact role of these DNA alterations in modulating estrogen receptor α (ERα) machinery remains elusive. The present study aimed to analyze the impact of DNA damage agents on ERα signaling in breast cancer cells and assess the signaling pathways mediating the influence of DNA damage drugs on the ERα machinery. Cell viability was assessed using the MTT method, while the expression of signaling proteins was analyzed by immunoblotting. ERα activity in the cells treated with various drugs (17β-estradiol, tamoxifen, 5-fluorouracil) was assessed through reporter gene assays. In vitro experiments were conducted on MCF7 breast cancer cells subjected to varying durations of 5-fluorouracil (5-FU) treatment. Two distinct cell responses to 5-FU were identified based on the duration of the treatment. A singular dose of 5-FU induces pronounced DNA fragmentation, temporally suppressing ERα signaling while concurrently activating AKT phosphorylation. This suppression reverses upon 5-FU withdrawal, restoring normalcy within ten days. However, chronic 5-FU treatment led to the emergence of 5-FU-resistant cells with irreversible alterations in ERα signaling, resulting in partial hormonal resistance. These changes mirror those observed in cells subjected to UV-induced DNA damage, underscoring the pivotal role of DNA damage in shaping estrogen signaling alterations in breast cancer cells. In summary, the results of the present study suggested that the administration of DNA damage agents to cancer cells can trigger irreversible suppression of estrogen signaling, fostering the development of partial hormonal resistance. This outcome may ultimately impede the efficacy of combined or subsequent chemo- and hormone therapy strategies.
Keywords: 5-fluorouracil; DNA damage; breast cancer; estrogen receptor α; progression; resistance; tamoxifen; ultraviolet C irradiation.
Copyright: © Scherbakov et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




Similar articles
-
Epigenetic reactivation of estrogen receptor-α (ERα) by genistein enhances hormonal therapy sensitivity in ERα-negative breast cancer.Mol Cancer. 2013 Feb 4;12:9. doi: 10.1186/1476-4598-12-9. Mol Cancer. 2013. PMID: 23379261 Free PMC article.
-
The phenomenon of acquired resistance to metformin in breast cancer cells: The interaction of growth pathways and estrogen receptor signaling.IUBMB Life. 2016 Apr;68(4):281-92. doi: 10.1002/iub.1481. Epub 2016 Feb 19. IUBMB Life. 2016. PMID: 26892736
-
AKT3 regulates ErbB2, ErbB3 and estrogen receptor α expression and contributes to endocrine therapy resistance of ErbB2(+) breast tumor cells from Balb-neuT mice.Cell Signal. 2014 May;26(5):1021-9. doi: 10.1016/j.cellsig.2014.01.018. Epub 2014 Jan 24. Cell Signal. 2014. PMID: 24463007
-
Towards Unravelling the Role of ERα-Targeting miRNAs in the Exosome-Mediated Transferring of the Hormone Resistance.Molecules. 2021 Nov 3;26(21):6661. doi: 10.3390/molecules26216661. Molecules. 2021. PMID: 34771077 Free PMC article.
-
Poly-ADP-Ribosylation of Estrogen Receptor-Alpha by PARP1 Mediates Antiestrogen Resistance in Human Breast Cancer Cells.Cancers (Basel). 2019 Jan 4;11(1):43. doi: 10.3390/cancers11010043. Cancers (Basel). 2019. PMID: 30621214 Free PMC article.
References
-
- Khan M, Rasul A, Yi F, Zhong L, Ma T. Jaceosidin induces p53-dependent G2/M phase arrest in U87 glioblastoma cells. Asian Pac J Cancer Prev. 2011;12:3235–3238. - PubMed
-
- Rasul A, Khan M, Yu B, Ma T, Yang H. Xanthoxyletin, a coumarin induces S phase arrest and apoptosis in human gastric adenocarcinoma SGC-7901 cells. Asian Pac J Cancer Prev. 2011;12:1219–1223. - PubMed
-
- Chrienova Z, Rysanek D, Oleksak P, Stary D, Bajda M, Reinis M, Mikyskova R, Novotny O, Andrys R, Skarka A, et al. Discovery of small molecule mechanistic target of rapamycin inhibitors as anti-aging and anti-cancer therapeutics. Front Aging Neurosci. 2022;14(1048260) doi: 10.3389/fnagi.2022.1048260. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
