Effects of Helicobacter pylori infection on intestinal microbiota, immunity and colorectal cancer risk
- PMID: 38343887
- PMCID: PMC10853882
- DOI: 10.3389/fcimb.2024.1339750
Effects of Helicobacter pylori infection on intestinal microbiota, immunity and colorectal cancer risk
Abstract
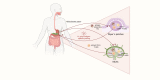
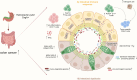
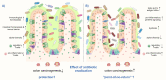
Infecting about half of the world´s population, Helicobacter pylori is one of the most prevalent bacterial infections worldwide and the strongest known risk factor for gastric cancer. Although H. pylori colonizes exclusively the gastric epithelium, the infection has also been associated with various extragastric diseases, including colorectal cancer (CRC). Epidemiological studies reported an almost two-fold increased risk for infected individuals to develop CRC, but only recently, direct causal and functional links between the chronic infection and CRC have been revealed. Besides modulating the host intestinal immune response, H. pylori is thought to increase CRC risk by inducing gut microbiota alterations. It is known that H. pylori infection not only impacts the gastric microbiota at the site of infection but also leads to changes in bacterial colonization in the distal large intestine. Considering that the gut microbiome plays a driving role in CRC, H. pylori infection emerges as a key factor responsible for promoting changes in microbiome signatures that could contribute to tumor development. Within this review, we want to focus on the interplay between H. pylori infection, changes in the intestinal microbiota, and intestinal immunity. In addition, the effects of H. pylori antibiotic eradication therapy will be discussed.
Keywords: Helicobacter pylori; antibiotics; colorectal cancer; eradication therapy; immune response; intestinal microbiome.
Copyright © 2024 Engelsberger, Gerhard and Mejías-Luque.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

