Long-term Follow-up and Safety of Patients after an Upfront Therapy with Letrozole for Early Breast Cancer in Routine Clinical Care - The PreFace Study
- PMID: 38344045
- PMCID: PMC10853028
- DOI: 10.1055/a-2238-3153
Long-term Follow-up and Safety of Patients after an Upfront Therapy with Letrozole for Early Breast Cancer in Routine Clinical Care - The PreFace Study
Erratum in
-
Correction: Long-term Follow-up and Safety of Patients after an Upfront Therapy with Letrozole for Early Breast Cancer in Routine Clinical Care - The PreFace Study.Geburtshilfe Frauenheilkd. 2024 Apr 30;84(2):e10. doi: 10.1055/a-2314-3693. eCollection 2024 Feb. Geburtshilfe Frauenheilkd. 2024. PMID: 38690326 Free PMC article.
Abstract
Introduction: Adjuvant treatment of patients with early-stage breast cancer (BC) should include an aromatase inhibitor (AI). Especially patients with a high recurrence risk might benefit from an upfront therapy with an AI for a minimum of five years. Nevertheless, not much is known about the patient selection for this population in clinical practice. Therefore, this study analyzed the prognosis and patient characteristics of postmenopausal patients selected for a five-year upfront letrozole therapy.
Patients and methods: From 2009 to 2011, 3529 patients were enrolled into the adjuvant phase IV PreFace clinical trial (NCT01908556). Postmenopausal hormone receptor-positive BC patients, for whom an upfront five-year therapy with letrozole (2.5 mg/day) was indicated, were eligible. Disease-free survival (DFS), overall survival (OS) and safety in relation to patient and tumor characteristics were assessed.
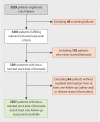
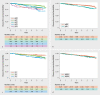
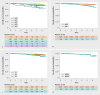
Results: 3297 patients started letrozole therapy. The majority of patients (n = 1639, 57%) completed the five-year treatment. 34.5% of patients continued with endocrine therapy after the mandated five-year endocrine treatment. Five-year DFS rates were 89% (95% CI: 88-90%) and five-year OS rates were 95% (95% CI: 94-96%). In subgroup analyses, DFS rates were 83%, 84% and 78% for patients with node-positive disease, G3 tumor grading, and pT3 tumors respectively. The main adverse events (any grade) were pain and hot flushes (66.8% and 18.3% of patients).
Conclusions: The risk profile of postmenopausal BC patients selected for a five-year upfront letrozole therapy showed a moderate recurrence and death risk. However, in subgroups with unfavorable risk factors, prognosis warrants an improvement, which might be achieved with novel targeted therapies.
Einleitung: Die adjuvante Behandlung von Patientinnen mit Brustkrebs im Frühstadium sollte eine Therapie mit einem Aromatasehemmer (AH) miteinschließen. Patientinnen mit einem hohen Rezidivrisiko profitieren besonders von einer Upfront-Therapie mit einem AH, die sich über einen Mindestzeitraum von 5 Jahren erstreckt. Dennoch ist nicht viel über die Selektion geeigneter Patientinnen in dieser Population in der Praxis bekannt. Diese Studie hat deshalb die Prognosen und Charakteristika von postmenopausalen Patientinnen, die für eine Upfront-Therapie mit Letrozol über 5 Jahre ausgewählt wurden, analysiert.
Patientinnen und methoden: Zwischen 2009 und 2011 nahmen 3529 Patientinnen an der adjuvanten klinischen Phase-IV-PreFace-Studie (NCT01908556) teil. Eingeschlossen wurden postmenopausale hormonrezeptorpositive Brustkrebspatientinnen mit Indikation für eine 5-jährige Upfront-Therapie mit Letrozol (2,5 mg/Tag). Beurteilt wurden krankheitsfreies Überleben (KFÜ), Gesamtüberleben (GÜ) und Sicherheit in Abhängigkeit von den Patientinnen- und Tumorcharakteristika.
Ergebnisse: Insgsamt begannen 3297 Patientinnen mit einer Letrozol-Therapie. Die Mehrheit der Patientinnen (n = 1639, 57%) haben die 5-jährige Behandlung abgeschlossen. Nach Beendigung der angeordneten 5-jährigen endokrinen Behandlung machten 34,5% der Patientinnen mit einer endokrinen Therapie weiter. Die 5-jährige KFÜ-Rate betrug 89% (95%-KI: 88–90%) und die 5-jährige GÜ-Rate war 95% (95%-KI: 94–96%). Bei der Subgruppenanalyse betrugen die KFÜ-Raten 83%, 84% resp. 78% für Patientinnen mit jeweils nodal-positivem Brustkrebs, Tumorgrad G3 bzw. pT3-Tumoren. Zu den wichtigsten unerwünschten Ereignissen (aller Schweregrade) gehörten Schmerzen sowie Hitzewallungen (die jeweils bei 66,8% bzw. 18,3% der Patientinnen auftraten).
Schlussfolgerungen: Die Analyse des Risikoprofils von postmenopausalen Brustkrebspatientinnen, die für eine 5-jährige Upfront-Therapie mit Letrozol ausgewählt wurden, zeigte ein mäßiges Rezidiv- und Sterberisiko. Aber bei Untergruppen mit ungünstigen Risikofaktoren rechtfertigt die Prognose die Suche nach Verbesserungen, die mithilfe neuartiger zielgerichteter Therapien erreicht werden können.
Keywords: aromatase inhibitors; early breast cancer; hormone therapy; letrozole; prognosis.
The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/).
Conflict of interest statement
Conflict of Interest P. G. received honoraria from Novartis, MSD, and AstraZeneca. K. A. received speaker honoraria from Roche Pharma AG, Pfizer Pharma GmbH and AstraZeneca. C. C. H. received honoraria from Roche, Pfizer, Novartis, AstraZeneca, Gilead, Daiichi Sankyo, Eisai, Gilead and MSD, and received travel grants from Daiichi Sankyo. B. A. received honoraria from AstraZeneca, Gilead, Genomic Health, Roche, Novartis, Celgene, Lilly, MSD, Eisai, Stemline, Teva, Tesaro, Daiichi Sankyo and Pfizer. Received travel grants from AstraZeneca, Roche, Novartis, Celgene, Lilly, Eisai, Stemline, Daiichi Sankyo and Pfizer. Participated in the data safety monitoring board or advisory boards for AstraZeneca, Gilead, Genomic Health, Roche, Novartis, Celgene, Lilly, MSD, Eisai, Tesaro, Daiichi Sankyo and Pfizer. S. K. received honoraria from Amgen, Celgene, Daiichi Sankyo, Novartis and Roche. C. T. received honoraria for advisory boards and lectures from Amgen, AstraZeneca, Celgene, Daiichi Sankyo, Eisai, Gilead, Lilly, MSD, Mylan, Nanostring, Novartis, Pfizer, Pierre Fabre, Puma, Roche, Seagen, Vifor. H.-C. K. has received honoraria from Pfizer, Novartis, Roche, Genomic Health/Exact Sciences, Amgen, AstraZeneca, Riemser, Carl Zeiss Meditec, Teva, Theraclion, Janssen-Cilag, GSK, LIV Pharma, Lilly, Daiichi Sankyo, Gilead, Zuellig, travel support from Carl Zeiss Meditec, LIV Pharma, Novartis, Amgen, Pfizer, Daiichi Sankyo, Tesaro, Gilead, AstraZeneca, Zuellig, Stemline, participated in data safety monitoring board or advisory boards for Pfizer, Novartis, SurgVision, Carl Zeiss Meditec, Amgen, Onkowissen, MSD, Gilead, Daiichi Sankyo, Seagen, Genomic Health/Exact Sciences, Agendia, Lilly and owns stock of Theraclion SA. W. J. has received research grants and/or honoraria from Sanofi-Aventis, Daiichi Sankyo, Novartis, Roche, Pfizer, Lilly, AstraZeneca, Chugai, GSK, Eisai, Cellgene and Johnson & Johnson. A. S. reported grants from Celgene, Roche and AbbVie. Personal fees from Cellgene, Roche, Pfizer, AstraZeneca, Novartis, MSD, Tesaro, Lilly, Seagen, Gilead, GSK, Bayer, Amgen, and Pierre Fabre, and travel grants from Celgene, Roche, Pfizer and AstraZeneca. F. M. received honoraria from Amgen, AstraZeneca, Celgene, Clovis Oncology, CureVac, Eisai, Genomic Health, GlaxoSmithKline, Immunomedics, Janssen-Cilag, Lilly, MSD, Novartis, Pfizer, PharmaMar, Roche, Seattle Genetics, Tesaro. M. W. S. received honoraria from AstraZeneca, Pfizer, Clovis, Mylan, Roche, Gedeon Richter, Carl Zeiss Meditec, travel support from Pfizer, Carl Zeiss Meditec. C. J. reports personal fees from AstraZeneca, Exact Sciences, Lilly, Novartis and Roche. V. M. received speaker honoraria from Amgen, AstraZeneca, Daiichi Sankyo, Eisai, GSK, Pfizer, MSD, Medac, Novartis, Roche, Teva, Seagen, Onkowissen, high5 Oncology, Medscape, Gilead. Consultancy honoraria from Hexal, Roche, Pierre Fabre, Amgen, ClinSol, Novartis, MSD, Daiichi Sankyo, Eisai, Lilly, Sanofi, Seagen, Gilead. Institutional research support from Novartis, Roche, Seagen, Genentech. Travel grants: Roche, Pfizer, Daiichi Sankyo. E. B. received honoraria from Novartis, Hexal, BMS, Lilly, Pfizer, Roche, MSD, Bayer, Ipsen, Bluebird, Braun and onkowissen.de for consulting, clinical research management or medical education activities. S. Y. B. has received honoraria from Roche Pharma, Novartis, Pfizer, MSD, Teva, AstraZeneca. T. N. F. has received honoraria from Novartis, Roche, Pfizer, Teva, Daiichi Sankyo, AstraZeneca and MSD. P. A. F. reports personal fees from Novartis, grants from Biontech, personal fees from Pfizer, personal fees from Daiichi Sankyo, personal fees from AstraZeneca, personal fees from Eisai, personal fees from MSD, grants from Cepheid, personal fees from Lilly, personal fees from Pierre Fabre, personal fees from SeaGen, personal fees from Roche, personal fees from Hexal, personal fees from Agendia, personal fees from Gilead. C.R. received honoraria from MSD and AstraZeneca, travel expenses from the Swiss Society of Senology and the Swiss Society of Gynecology. All of the remaining authors declare that they do not have any conflicts of interest.
Figures
References
-
- National Comprehensive Cancer Network (NCCN) . NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) Breast Cancer Version 3.2022. 2022. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf

