Treatment in Certified Breast Cancer Centers Improves Chances of Survival of Patients with Breast Cancer: Evidence Based on Health Care Data from the WiZen Study
- PMID: 38344046
- PMCID: PMC10853029
- DOI: 10.1055/a-1869-1772
Treatment in Certified Breast Cancer Centers Improves Chances of Survival of Patients with Breast Cancer: Evidence Based on Health Care Data from the WiZen Study
Abstract
Introduction: Certified breast cancer centers offer specific quality standards in terms of their structure, diagnostic and treatment approaches with regards to breast surgery, drug-based cancer therapy, radiotherapy, and psychosocial support. Such centers aim to improve treatment outcomes of breast cancer patients. The question investigated here was whether patients with primary breast cancer have a longer overall survival if they are treated in a certified breast cancer center compared to treatment outside these centers.
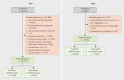
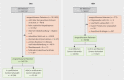
Methods: We used patient-specific data (demographics, diagnoses, treatments) obtained from data held by mandatory health insurance companies ( gesetzliche Krankenversicherung , GKV) and clinical cancer registries (KKR) for the period 2009-2017 as well as hospital characteristics recorded in standardized quality reports. Using multivariable Cox regression analysis, we investigated differences in survival between patients treated in hospitals certified as breast cancers centers by the German Cancer Society (DKG) and patients treated in hospitals which had not been certified by the DKG.
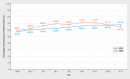
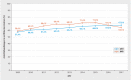
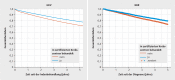
Results: The sample population consisted of 143720 (GKV data) and 59780 (KKR data) patients with breast cancer, who were treated in 1010 hospitals across Germany (280 DKG-certified, 730 not DKG-certified). 63.5% (GKV data) and 66.7% (KKR data) of patients, respectively, were treated in DKG-certified breast cancer centers. Cox regression analysis for overall survival which included patient and hospital characteristics found a significantly lower mortality risk for patients treated in DKG-certified breast cancer centers (GKV data: HR = 0.77, 95% CI = 0.74-0.81; KKR data: HR = 0.88, 95% CI = 0.85-0.92). This result remained stable even after several sensitivity analyses including stratified estimates for subgroups of patients and hospitals. The effect was even more pronounced for recurrence-free survival (KKR data: HR = 0.78, 95% CI = 0.74-0.82).
Conclusions: Patients who are treated by an interdisciplinary team in a DKG-certified breast cancer had clear and statistically significantly better survival rates. Certification is therefore an effective means of improving the quality of care, and more patients should be treated in certified breast cancer centers.
Keywords: breast cancer; certification; health care research; health care-related data; outcome quality.
The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/).
Conflict of interest statement
Conflict of Interest OS, VB, CB and JS work in a university hospital with certified cancer centers, and MR previously also worked there. In addition, they received grants from the Innovation Fund of the Federal Joint Committee when carrying out the study. Independently of this study, JS received institutional grants for investigator-initiated research from the G-BA, the BMG, BMBF, the EU, the German Federal State of Saxony, Novartis, Sanofi, ALK and Pfizer. He also took part in Advisory Board Meetings as a paid consultant for Sanofi, Lilly and the ALK. Independently of this study, OS was a paid consultant for Novartis. He is also a member of the certification committee “Skin Cancer Centers” of the German Cancer Society and a member of the panel of experts for the project “Research into criteria to evaluate certificates and quality seals in accordance with Sec. 137a para. 3 sentence 2 No. 7 SGB V” for the Institute for Quality Assurance and Transparency in Healthcare (IQTIG). PW heads the DKG-certified Breast and Gynecological Cancer Center at the university hospital of Dresden University of Technology and is an additional member of the Board of Directors of NCT Dresden. PW is receiving institutional grants for investigator-initiated research from the DFG, Krebshilfe, Sächsische Aufbaubank (SAB), Gynäko-Onkologische Forschungsstiftung, Amgen, AstraZeneca, MSD, Novartis, Pfizer, Roche, Clovis, GSK. PW receives honoraria as an Advisory Board member for Amgen, AstraZeneca, MSD, Novartis, Pfizer, Lilly, Roche, Teva, Eisai, Gilead, GSK and Daiichi Sankyo. AS is the speaker for the Certification Committee for Breast Cancer Centers of the DKG and for many years he was the director of gynecological hospitals with certified breast cancer centers. He has had no financially relevant cooperations with industry in the last 4 years. ECI and OO work in a certified breast cancer center, gynecological cancer center and oncological center. OO is a member of the Executive Board of the German Cancer Society, head of the University Cancer Center Regensburg and a member of the Board of Directors of CCC WERA. TL works in a certified breast and gynecological cancer center. TL has received honoraria (lectures/consultancy work/travel costs) from Novartis, Roche, Amgen, GSK, Pfizer, Gilead, Daiichi Sankyo, AstraZeneca, Lilly, Myriad, MSD and Esai. TP is head of a DKG-certified breast and gynecological cancer center. St. Marien Amberg Medical Center is also an oncology center according to the criteria of the DKG. The other authors state that they have no conflict of interest.
Figures

References
-
- Zentrum für Krebsregisterdaten im Robert Koch-Institut . Datenbankabfrage mit Schätzung der Inzidenz, Prävalenz und des Überlebens von Krebs in Deutschland auf Basis der epidemiologischen Landeskrebsregisterdaten. - DOI
-
- GEKID ; ZfKD . Berlin: Robert Koch-Institut; 2021. Krebs in Deutschland für 2017/2018.
-
- DKG (Deutsche Krebsgesellschaft) . Berlin: DKG (Deutsche Krebsgesellschaft); 2022. Jahresbericht der zertifizierten Brustkrebszentren – Auditjahr 2021/Kennzahlenjahr 2020.
LinkOut - more resources
Full Text Sources

