No additional risk of congenital anomalies after first-trimester dydrogesterone use: a systematic review and meta-analysis
- PMID: 38344249
- PMCID: PMC10859181
- DOI: 10.1093/hropen/hoae004
No additional risk of congenital anomalies after first-trimester dydrogesterone use: a systematic review and meta-analysis
Abstract
Study question: Is exposure to dydrogesterone a risk factor for congenital anomalies when given in the first trimester for recurrent/threatened pregnancy loss or as luteal support in assisted reproductive technology (ART)?
Summary answer: Dydrogesterone, when given in the first trimester for recurrent/threatened pregnancy loss or as luteal support in ART, is not a relevant additional risk factor for congenital anomalies.
What is known already: Despite large clinical trials and meta-analyses that show no association between dydrogesterone and congenital anomalies, some recently retracted publications have postulated an association with teratogenicity. Dydrogesterone is also often rated as less safe than bioidentical progestins.
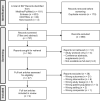
Study design size duration: A systematic review was conducted according to a pre-specified protocol with searches on Medline, Embase, Cochrane Central Register of Controlled Trials (CENTRAL), and Clinicaltrials.gov. The search was limited to human studies, with no restrictions on language, geographical region, or date. The search algorithm used a PICO (Population, Intervention, Comparison, Outcome)-style approach combining both simple search terms and medical subject heading terms. As congenital anomalies are mostly reported as secondary outcomes, the search term 'safety' was added.
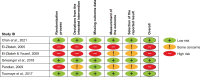
Participants/materials setting methods: Interventional study and observational study (OS) designs were eligible for inclusion. Inclusion criteria were: women >17 years old treated for threatened miscarriage, recurrent pregnancy loss, and/or ART; the use of dydrogesterone in the first trimester compared with placebo, no treatment or other interventions; and reporting of congenital anomalies in newborns or infants ≤12 months old (primary outcome). Two authors (A.K., M.R.N.) independently extracted the following data: general study information, study population details, intervention and comparator(s), and frequencies of congenital anomalies (classification, time of determination, and type). Risk of bias focused on the reporting of congenital malformations and was assessed using the Cochrane Risk of Bias Tool Version 2 or the ROBINS-I tool. The GRADEproGDT platform was used to generate the GRADE summary of findings table.
Main results and the role of chance: Of the 897 records retrieved during the literature search, 47 were assessed for eligibility. Nine studies were included in the final analysis: six randomized controlled trials (RCTs) and three OSs. Among the RCTs, three had a low risk and three a high risk of bias. Two of the OSs were considered to have a serious risk of bias and one with critical risk of bias and was excluded for the evidence syntheses. The eight remaining studies included a total of 5070 participants and 2680 live births from 16 countries. In the meta-analysis of RCTs only, the overall risk ratio (RR) was 0.92 [95% CI 0.55; 1.55] with low certainty. When the two OSs were included, the overall RR was 1.11 [95% CI 0.73; 1.68] with low certainty.
Limitations reasons for caution: The studies included in the analysis do not report congenital anomalies as the primary outcome; reporting of congenital anomalies was often not standardized.
Wider implications of the findings: This systematic literature review and meta-analysis provide clear reassurance to both clinicians and patients that dydrogesterone is not associated with congenital anomalies above the rate that might be expected due to environmental and genetic factors. The results of this work represent the highest current level of evidence for the question of congenital anomalies, which removes the existing uncertainty caused by poor quality and retracted studies.
Study funding/competing interests: Editorial support was provided by Highfield Communication Consultancy, Oxford, UK, sponsored by Abbott Products Operations AG, Allschwil, Switzerland. A.K., J.A.G.-V., L.P.S., J.N.v.d.A., and J.F.S. received honoraria from Abbott for preparation and participation in an advisory board. J.A.G.-V. received grants and lecture fees from Merck, Organon, Ferring, Gedeon Richter, and Theramex. M.R.N. has no conflicts of interest. J.N.v.d.A. and J.A.G.-V. have no other conflicts of interest. A.K. received payment from Abbott for a talk at the IVF Worldwide congress on 22 September 2023. J.F.S. has received grants from the National Institutes of Health, royalties/licences from Elsevier and Prescient Medicine (SOLVD Health), consulting fees from Burroughs Wellcome Fund (BWF) and Bayer, honoraria from Magee Women's Research Institute, Wisconsin National Primate Research Centre, University of Kansas and Oakridge National Research Laboratory, Agile, Daiichi Sankyo/American Regent, and Bayer, and travel support to attend meetings for the International Academy of Human Reproduction (IAHR). J.F.S. has patents related to diagnosis and treatment of PCOS and prediction of preterm birth. J.F.S. participates on advisory boards for SOLVD Health, Wisconsin National Primate Research Centre, and FHI360, was the past President board member of the Society for Reproductive Investigation, has a leadership role for the following organizations: Scientific Advisory Board, SOLVD Health, EAB Chair for contraceptive technology initiative, FHI360, EAB member, Wisconsin National Primate Research Centre, Advisory Board for MWRI Summit, Chair of BWF NextGen Pregnancy Research Panel, Medical Executive Committee at the Howard, and Georgeanna Jones Foundation, and is Vice President, IAHR. L.P.S. has received consulting fees from Shield Pharmaceuticals, Scynexis, Organon, Natera, Celula China, AiVF, Agile, Daiichi Sankyo, American Regent, and Medicem, honoraria from Agile, Daiichi Sankyo/American Regent, and Bayer, and travel support from BD Diagnostics. L.P.S. participates on the data safety monitoring board for Astellas and is a Chair of DSMB for fezolinetant. Abbott played no role in the funding of the study or in study design, data collection, data analysis, data interpretation, or writing of the report.
Trial registration number: PROSPERO 2022 CRD42022356977.
Keywords: ART; congenital abnormality; meta-analysis; miscarriage; progesterone; recurrent miscarriage.
© The Author(s) 2024. Published by Oxford University Press on behalf of European Society of Human Reproduction and Embryology.
Conflict of interest statement
A.K., J.A.G.-V., L.P.S., J.N.v.d.A., and J.F.S. received honoraria from Abbott for preparation and participation in an advisory board. J.A.G.-V. received grants and lecture fees from Merck, Organon, Ferring, Gedeon Richter, and Theramex. M.R.N. has no conflicts of interest. J.N.v.d.A. and J.A.G.-V. have no other conflicts of interest. A.K. received payment from Abbott for a talk at the IVF Worldwide congress on 22 September 2023. J.F.S. has received grants from the National Institutes of Health, royalties/licences from Elsevier and Prescient Medicine (SOLVD Health), consulting fees from Burroughs Wellcome Fund (BWF) and Bayer, honoraria from Magee Women's Research Institute, Wisconsin National Primate Research Centre, University of Kansas and Oakridge National Research Laboratory, Agile, Daiichi Sankyo/American Regent, and Bayer, and travel support to attend meetings for the International Academy of Human Reproduction (IAHR). J.F.S. has patents related to diagnosis and treatment of PCOS and prediction of preterm birth. J.F.S. participates on advisory boards for SOLVD Health, Wisconsin National Primate Research Centre, and FHI360, was the past President board member of the Society for Reproductive Investigation, has a leadership role for the following organizations: Scientific Advisory Board, SOLVD Health, EAB Chair for contraceptive technology initiative, FHI360, EAB member, Wisconsin National Primate Research Centre, Advisory Board for MWRI Summit, Chair of BWF NextGen Pregnancy Research Panel, Medical Executive Committee at the Howard, and Georgeanna Jones Foundation, and is Vice President, IAHR. L.P.S. has received consulting fees from Shield Pharmaceuticals, Scynexis, Organon, Natera, Celula China, AiVF, Agile, Daiichi Sankyo, American Regent, and Medicem, honoraria from Agile, Daiichi Sankyo/American Regent, and Bayer, and travel support from BD Diagnostics. L.P.S. participates on the data safety monitoring board for Astellas and is a Chair of DSMB for fezolinetant. Abbott played no role in the funding of the study or in study design, data collection, data analysis, data interpretation, or writing of the report.
Figures


Comment in
-
Absence of evidence is not evidence of absence for first trimester dydrogesterone-induced birth defects.Hum Reprod Open. 2024 May 11;2024(2):hoae030. doi: 10.1093/hropen/hoae030. eCollection 2024. Hum Reprod Open. 2024. PMID: 38784056 Free PMC article. No abstract available.
-
Reply: Absence of evidence is not evidence of absence for first trimester dydrogesterone-induced birth defects.Hum Reprod Open. 2024 May 11;2024(2):hoae031. doi: 10.1093/hropen/hoae031. eCollection 2024. Hum Reprod Open. 2024. PMID: 38784057 Free PMC article. No abstract available.
Similar articles
-
Evidence-based guideline: premature ovarian insufficiency.Hum Reprod Open. 2024 Dec 9;2024(4):hoae065. doi: 10.1093/hropen/hoae065. eCollection 2024. Hum Reprod Open. 2024. PMID: 39660328 Free PMC article.
-
Evidence-based guideline: Premature Ovarian Insufficiency.Fertil Steril. 2025 Feb;123(2):221-236. doi: 10.1016/j.fertnstert.2024.11.007. Epub 2024 Dec 9. Fertil Steril. 2025. PMID: 39652037
-
Evidence-based guideline: unexplained infertility†.Hum Reprod. 2023 Oct 3;38(10):1881-1890. doi: 10.1093/humrep/dead150. Hum Reprod. 2023. PMID: 37599566 Free PMC article.
-
Current global status of male reproductive health.Hum Reprod Open. 2024 Apr 12;2024(2):hoae017. doi: 10.1093/hropen/hoae017. eCollection 2024. Hum Reprod Open. 2024. PMID: 38699533 Free PMC article. Review.
-
Safety of fertility preservation techniques before and after anticancer treatments in young women with breast cancer: a systematic review and meta-analysis.Hum Reprod. 2022 May 3;37(5):954-968. doi: 10.1093/humrep/deac035. Hum Reprod. 2022. PMID: 35220429 Free PMC article.
Cited by
-
Absence of evidence is not evidence of absence for first trimester dydrogesterone-induced birth defects.Hum Reprod Open. 2024 May 11;2024(2):hoae030. doi: 10.1093/hropen/hoae030. eCollection 2024. Hum Reprod Open. 2024. PMID: 38784056 Free PMC article. No abstract available.
-
Birth defects reporting and the use of dydrogesterone: a disproportionality analysis from the World Health Organization pharmacovigilance database (VigiBase).Hum Reprod Open. 2025 Jan 2;2025(1):hoae072. doi: 10.1093/hropen/hoae072. eCollection 2025. Hum Reprod Open. 2025. PMID: 39807112 Free PMC article.
-
Why does the latest pharmacovigilance data not reflect clinical experience with dydrogesterone?Hum Reprod Open. 2025 May 26;2025(3):hoaf030. doi: 10.1093/hropen/hoaf030. eCollection 2025. Hum Reprod Open. 2025. PMID: 40497085 Free PMC article. No abstract available.
-
Unmasking the risk: clinical trials versus real-world evidence on dydrogesterone and birth defects.Hum Reprod Open. 2024 Dec 24;2025(1):hoae073. doi: 10.1093/hropen/hoae073. eCollection 2025. Hum Reprod Open. 2024. PMID: 39807113 Free PMC article. No abstract available.
-
Oral dydrogesterone along with vaginal micronized progesterone supplementation for luteal phase support in IVF patients, and its impact on pregnancy and live birth rates: a prospective randomized trial.BMC Pregnancy Childbirth. 2024 Dec 21;24(1):845. doi: 10.1186/s12884-024-07069-8. BMC Pregnancy Childbirth. 2024. PMID: 39709390 Free PMC article. Clinical Trial.
References
-
- Babalioğlu R, Varol FG, Ilhan R, Yalçin O, Cizmecioğlu F.. Progesterone profiles in luteal-phase defects associated with recurrent spontaneous abortions. J Assist Reprod Genet 1996;13:306–309. - PubMed
-
- Barbosa MW, Silva LR, Navarro PA, Ferriani RA, Nastri CO, Martins WP.. Dydrogesterone vs progesterone for luteal-phase support: systematic review and meta-analysis of randomized controlled trials. Ultrasound Obstet Gynecol 2016;48:161–170. - PubMed
-
- Baron J, Holzman GB, Schulkin J.. Attitudes of obstetricians and gynecologists toward hormone replacement therapy. Med Decis Making 1998;18:406–411. - PubMed
-
- Carp HJA. Progestogens and pregnancy loss. Climacteric 2018;21:380–384. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
