A Phase 2, Prospective, Multicenter, Single-Arm Trial of Transarterial Chemoembolization Therapy in Combination Strategy with Lenvatinib in Patients with Unresectable Intermediate-Stage Hepatocellular Carcinoma: TACTICS-L Trial
- PMID: 38344448
- PMCID: PMC10857829
- DOI: 10.1159/000531377
A Phase 2, Prospective, Multicenter, Single-Arm Trial of Transarterial Chemoembolization Therapy in Combination Strategy with Lenvatinib in Patients with Unresectable Intermediate-Stage Hepatocellular Carcinoma: TACTICS-L Trial
Abstract
Introduction: Transarterial chemoembolization (TACE) is the standard treatment for unresectable intermediate-stage hepatocellular carcinoma (HCC), but recurrence after TACE is common. The present phase 2, prospective, multicenter, single-arm trial, the TACTICS-L trial, investigated the efficacy and safety of TACE plus lenvatinib (LEN), a drug that more strongly promotes vascular normalization and has a better objective response rate (ORR) than sorafenib (jRCTs031180074).
Methods: Participants were patients with HCC who had not previously received systemic therapy, hepatic arterial infusion chemotherapy, or immunotherapy and who were ineligible for resection or percutaneous ablation therapy. LEN was to be administered 14-21 days before the first TACE, stopped 2 days before TACE, and resumed 3 days after TACE. Key inclusion criteria were unresectable HCC, Child-Pugh A liver function, 0-2 prior TACE sessions, tumor size ≤10 cm, number of tumors ≤10, and ECOG performance status 0-1. Key exclusion criteria were vascular invasion and extrahepatic spread. The primary endpoint was progression-free survival (PFS) by RECICL, and secondary endpoints were time to untreatable progression, ORR, overall survival (OS), and safety.
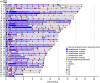
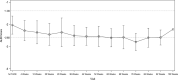
Results: A total of 62 HCC patients were enrolled in this trial. The median age was 72 years, 77.4% of patients were men, and 95.2% had PS 0. The primary endpoint of median PFS was 28.0 months (90% confidence interval [CI] 25.1-31.0) after a minimum 24 months of follow-up. The secondary endpoint of median OS was not reached (90% CI 35.5 months-NR). LEN-TACE achieved a high response rate and high complete response (CR) rate (4 weeks after the first TACE: ORR 79.0%, CR rate 53.2%; best response: ORR 88.7%, CR rate 67.7%) by RECICL. Exploratory subgroup analyses showed that the characteristics of responders/nonresponders (ORR and CR rate) were similar and that LEN-TACE would be effective in all subgroups, including the population in whom TACE alone would be less likely to be curative (e.g., patients with the non-simple nodular type or a high tumor burden). The relative dose intensity of LEN before the first TACE was important for achieving higher CR rate/ORR by LEN-TACE. No new safety concerns were observed.
Conclusion: The results of this trial provide encouraging evidence, supporting the efficacy and favorable safety profile of LEN-TACE in patients who are ineligible for locoregional therapy.
Keywords: Hepatocellular carcinoma; LEN-TACE sequential therapy; Lenvatinib; TACTICS-L trial; Transarterial chemoembolization.
© 2023 The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
Masatoshi Kudo: lecture: Eli Lilly, Bayer, Eisai, Chugai, Takeda, and AstraZeneca; grants: Gilead Sciences, Taiho, Otsuka, EA Pharma, AbbVie, Eisai, Chugai, and GE Healthcare; and advisory consulting: Chugai, Roche, AstraZeneca, and Eisai. Masatoshi Kudo is the Editor-in-Chief of Liver Cancer. Kazuomi Ueshima: consulting or advisory roles for Eisai, Lilly Japan, Pfizer, Chugai Pharma, and Takeda and honoraria from Bayer, Eisai, Lilly Japan, Chugai Pharma, Takeda, MSD, EA Pharma, Sumitomo Group, Taiho Pharmaceutical, and Kowa. Issei Saeki: honoraria from Eisai and research funding from Chugai Pharma. Toru Ishikawa, Nobukazu Tanabe, Yoshiyuki Wada, Masahiro Tsuda, Kazuhiko Nakao, Shunsuke Nojiri, Junji Furuse, Keisuke Hino, Chikara Ogawa, and Kenichi Yoshimura: no conflict of interest. Yoshitaka Inaba: Eisai, AstraZeneca, and Chugai. Naoki Morimoto: research funding from Eisai and AbbVie and honoraria from Eisai, AbbVie, and Chugai Pharma. Hiroshi Aikata: honoraria from Eisai and research funding from Eisai. Yasuteru Kondo: consulting or advisory roles for Terumo, Toray Medical, Hanaco Medical, UTM, Japan Lifeline, Century Medical, and Covidien. Takanori Ito: honoraria (speaker fee) from Chugai. Tetsuya Hosaka: honoraria from Gilead Sciences. Yusuke Kawamura: honoraria from Eisai, Chugai Pharma, and Terumo. Teiji Kuzuya: honoraria from Eisai, Takeda Yakuhin, Eli Lilly Japan, and Chugai Pharmaceutical. Hironori Koga: speaker honoraria from Eisai and Chugai Pharma and research grants from Shionogi, Daiichi Sankyo, and Chugai Pharma. Masafumi Ikeda: consulting or advisory roles for Eisai, Novartis, Chugai Pharma, Ono Pharmaceutical, AstraZeneca, Servier, and Takeda and honoraria from Taiho Pharmaceutical, Eisai, Lilly Japan, Chugai Pharma, AstraZeneca, and Servier. Michihisa Moriguchi: consulting or advisory roles for Bayer Yakuhin, Chugai Pharma, Eisai, and Lilly Japan and honoraria from Bayer Yakuhin, Chugai Pharma, Eisai, Lilly Japan, Takeda, and MSD. Takashi Hisai: employee of Eisai Co., Ltd. Yasuaki Arai: royalties from Sumitomo Bakelite and honoraria from Merit Medical Systems, Canon Medical Systems, Terumo International Systems, Sumitomo Bakelite, Boston Scientific, Japan Lifeline, Taiho Pharmaceutical, Guerbet Japan, AstraZeneca plc, Cosmotec, and Toray.
Figures


References
-
- Meyer T, Fox R, Ma YT, Ross PJ, James MW, Sturgess R, et al. Sorafenib in combination with transarterial chemoembolisation in patients with unresectable hepatocellular carcinoma (TACE 2): a randomised placebo-controlled, double-blind, phase 3 trial. Lancet Gastroenterol Hepatol. 2017;2(8):565–75. 10.1016/S2468-1253(17)30156-5. - DOI - PubMed
-
- Kudo M, Cheng AL, Park JW, Park JH, Liang PC, Hidaka H, et al. Orantinib versus placebo combined with transcatheter arterial chemoembolisation in patients with unresectable hepatocellular carcinoma (ORIENTAL): a randomised, double-blind, placebo-controlled, multicentre, phase 3 study. Lancet Gastroenterol Hepatol. 2018;3(1):37–46. 10.1016/S2468-1253(17)30290-X. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

