Chlorfenapyr Crystal Polymorphism and Insecticidal Activity
- PMID: 38344671
- PMCID: PMC10853905
- DOI: 10.1021/acs.cgd.3c01257
Chlorfenapyr Crystal Polymorphism and Insecticidal Activity
Abstract
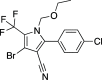
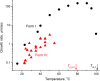
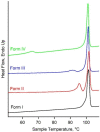
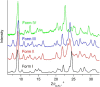
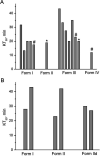
Four crystalline polymorphs of the proinsecticide chlorfenapyr [4-bromo-2-(4-chlorophenyl)-1-ethoxymethyl-5-trifluoromethyl-1H-pyrrole-3-carbonitrile] have been identified and characterized by polarized light optical microscopy, differential scanning calorimetry, Raman spectroscopy, X-ray diffraction, and electron diffraction. Three of the four structures were considered polytypic. Chlorfenapyr polymorphs show similar lethality against fruit flies (Drosophila melanogaster) and mosquitoes (Anopheles quadrimaculatus) with the least stable polymorph showing slightly higher lethality. Similar activities may be expected to be consistent with structural similarities. Knockdown kinetics, however, depend on an internal metabolic activating step, which further complicates polymorph-dependent bioavailability.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Darriet F. V.; Tho Vien N.; Robert V.; Carnevale P.. Evaluation of the efficacy of permethrin impregnated intact and perforated mosquito nets against vectors of malaria; World Health Organization: Geneva, 1984, WHO/VBC/84899, WHO/MAL/84.1008.
-
- Control of Neglected Tropical Diseases (NTD). Report of the twentieth WHOPES working group meeting WHO/HQ, Geneva 20–24 March 2017; Yadav R. S., Ed.; World Health Organization, 2017. https://www.who.int/publications/i/item/who-htm-ntd-whopes-2017.04 (accessed 2023-12-18).
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
