Bone marrow adipocytes fuel emergency hematopoiesis after myocardial infarction
- PMID: 38344689
- PMCID: PMC10857823
- DOI: 10.1038/s44161-023-00388-7
Bone marrow adipocytes fuel emergency hematopoiesis after myocardial infarction
Abstract
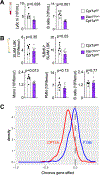
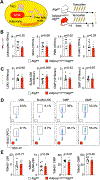
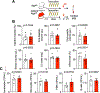
After myocardial infarction (MI), emergency hematopoiesis produces inflammatory myeloid cells that accelerate atherosclerosis and promote heart failure. Since the balance between glycolysis and mitochondrial metabolism regulates hematopoietic stem cell homeostasis, metabolic cues may influence emergency myelopoiesis. Here, we show in humans and female mice that hematopoietic progenitor cells increase fatty acid metabolism after MI. Blockade of fatty acid oxidation by deleting carnitine palmitoyltransferase (Cpt1A) in hematopoietic cells of Vav1Cre/+Cpt1Afl/fl mice limited hematopoietic progenitor proliferation and myeloid cell expansion after MI. We also observed reduced bone marrow adiposity in humans, pigs and mice following MI. Inhibiting lipolysis in adipocytes using AdipoqCreERT2Atglfl/fl mice or local depletion of bone marrow adipocytes in AdipoqCreERT2iDTR mice also curbed emergency hematopoiesis. Furthermore, systemic and regional sympathectomy prevented bone marrow adipocyte shrinkage after MI. These data establish a critical role for fatty acid metabolism in post-MI emergency hematopoiesis.
Keywords: Myocardial infarction; adipocytes; bone marrow; emergency hematopoiesis; fatty acid oxidation; lipolysis.
Conflict of interest statement
Competing interests statement All other authors declare no conflicts of interest, financial or otherwise.
Figures












Comment in
-
Bone marrow adipocytes support fatty acid metabolism during MI-mediated emergency haematopoiesis.Nat Rev Cardiol. 2024 Feb;21(2):74. doi: 10.1038/s41569-023-00987-8. Nat Rev Cardiol. 2024. PMID: 38114750 No abstract available.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
