Novel Treatment Paradigms: Primary IgA Nephropathy
- PMID: 38344739
- PMCID: PMC10851020
- DOI: 10.1016/j.ekir.2023.11.026
Novel Treatment Paradigms: Primary IgA Nephropathy
Abstract
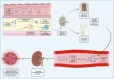
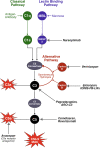
IgA nephropathy (IgAN) is the most common primary glomerulonephritis worldwide. Approximately 30% to 45% of patients progress to kidney failure (KF) within 20 to 25 years of diagnosis, and there has long been a lack of effective treatments. The therapeutic landscape in IgAN is rapidly evolving, driven in large part by the acceptance of the surrogate clinical trial end point of proteinuria reduction by regulatory authorities for the accelerated approval of new therapies. Two drugs, targeted release formulation (TRF)-budesonide (nefecon) and sparsentan, have recently been approved under this scheme. Advancing insights into the pathophysiology of IgAN, including the roles of the mucosal immune system, B-cells, the complement system, and the endothelin system have driven development of therapies that target these factors. This review outlines current, recently approved, and emerging therapies for IgAN.
Keywords: APRIL; BAFF; IgA nephropathy; clinical trials; complement; endothelin.
© 2023 International Society of Nephrology. Published by Elsevier Inc.
Figures
References
-
- Berger J., Hinglais N. [Intercapillary deposits of IgA-IgG] J Urol Nephrol (Paris) 1968;74:694–695. - PubMed
-
- D’Amico G., Colasanti G., Barbiano di Belgioioso G., et al. Long-term follow-up of IgA mesangial nephropathy: clinico-histological study in 374 patients. Semin Nephrol. 1987;7:355–358. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

