The Macrophage Landscape Across the Lifespan of a Human Cardiac Allograft
- PMID: 38344825
- PMCID: PMC11105989
- DOI: 10.1161/CIRCULATIONAHA.123.065294
The Macrophage Landscape Across the Lifespan of a Human Cardiac Allograft
Abstract
Background: Much of our knowledge of organ rejection after transplantation is derived from rodent models.
Methods: We used single-nucleus RNA sequencing to investigate the inflammatory myocardial microenvironment in human pediatric cardiac allografts at different stages after transplantation. We distinguished donor- from recipient-derived cells using naturally occurring genetic variants embedded in single-nucleus RNA sequencing data.
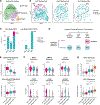
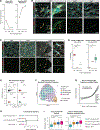
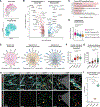
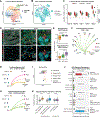
Results: Donor-derived tissue resident macrophages, which accompany the allograft into the recipient, are lost over time after transplantation. In contrast, monocyte-derived macrophages from the recipient populate the heart within days after transplantation and form 2 macrophage populations: recipient MP1 and recipient MP2. Recipient MP2s have cell signatures similar to donor-derived resident macrophages; however, they lack signatures of pro-reparative phagocytic activity typical of donor-derived resident macrophages and instead express profibrotic genes. In contrast, recipient MP1s express genes consistent with hallmarks of cellular rejection. Our data suggest that recipient MP1s activate a subset of natural killer cells, turning them into a cytotoxic cell population through feed-forward signaling between recipient MP1s and natural killer cells.
Conclusions: Our findings reveal an imbalance of donor-derived and recipient-derived macrophages in the pediatric cardiac allograft that contributes to allograft failure.
Keywords: allografts; heart; killer cells, natural; macrophages; pediatrics; tissue donor; transplant recipients.
Conflict of interest statement
Figures


Comment in
-
Leveraging Human Tissue for Discovery in Heart Transplantation.Circulation. 2024 May 21;149(21):1667-1669. doi: 10.1161/CIRCULATIONAHA.124.068884. Epub 2024 May 20. Circulation. 2024. PMID: 38768276 No abstract available.
References
-
- Epelman S, Lavine KJ, Beaudin AE, Sojka DK, Carrero JA, Calderon B, Brija T, Gautier EL, Ivanov S, Satpathy AT, Schilling JD, Schwendener R, Sergin I, Razani B, Forsberg EC, Yokoyama WM, Unanue ER, Colonna M, Randolph GJ, Mann DL. Embryonic and Adult-Derived Resident Cardiac Macrophages Are Maintained through Distinct Mechanisms at Steady State and during Inflammation. Immunity. 2014;40:91–104. - PMC - PubMed
-
- Zaman R, Hamidzada H, Kantores C, Wong A, Dick SA, Wang Y, Momen A, Aronoff L, Lin J, Razani B, Mital S, Billia F, Lavine KJ, Nejat S, Epelman S. Selective loss of resident macrophage-derived insulin-like growth factor-1 abolishes adaptive cardiac growth to stress. Immunity. 2021;54:2057–2071.e6. - PubMed
-
- Dick SA, Wong A, Hamidzada H, Nejat S, Nechanitzky R, Vohra S, Mueller B, Zaman R, Kantores C, Aronoff L, Momen A, Nechanitzky D, Li WY, Ramachandran P, Crome SQ, Becher B, Cybulsky MI, Billia F, Keshavjee S, Mital S, Robbins CS, Mak TW, Epelman S. Three tissue resident macrophage subsets coexist across organs with conserved origins and life cycles. Sci Immunol. 2022;7:eabf7777. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

