Influencing factors of clinical efficacy of roxadustat among hemodialysis patients
- PMID: 38345059
- PMCID: PMC10863536
- DOI: 10.1080/0886022X.2024.2308701
Influencing factors of clinical efficacy of roxadustat among hemodialysis patients
Abstract
Objective: To explore independent influencing factors for clinical efficacy of roxadustat in hemodialysis patients.
Methods: Hemodialysis patients treated with roxadustat were enrolled. The plasma trough concentrations of roxadustat were measured using liquid chromatography-tandem mass spectrometry (LC-MS/MS). A multiple logistic regression model was established to determine the factors that affect clinical efficacy of roxadustat in patients undergoing hemodialysis.
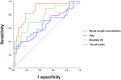
Results: A total of 67 hemodialysis patients were enrolled in the study. The results showed that age, blood trough concentration of roxadustat, and baseline hemoglobin (Hb) level were independent factors of clinical efficacy of roxadustat (OR = 1.06, p = .025 for age; OR = 1.001, p = .037 for plasma concentration; and OR = 0.941, p = .003 for baseline Hb), with an AUC score of 0.859.
Conclusions: Age, blood trough concentration of roxadustat, and baseline Hb level were independent influencing factors of the response to roxadustat in hemodialysis patients.
Keywords: Roxadustat; blood trough concentration of roxadustat; clinical efficacy; renal anemia.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures

Similar articles
-
Red blood cell lifespan in long-term hemodialysis patients treated with roxadustat or recombinant human erythropoietin.Ren Fail. 2021 Dec;43(1):1428-1436. doi: 10.1080/0886022X.2021.1988968. Ren Fail. 2021. PMID: 34657570 Free PMC article.
-
Roxadustat (FG-4592) Versus Epoetin Alfa for Anemia in Patients Receiving Maintenance Hemodialysis: A Phase 2, Randomized, 6- to 19-Week, Open-Label, Active-Comparator, Dose-Ranging, Safety and Exploratory Efficacy Study.Am J Kidney Dis. 2016 Jun;67(6):912-24. doi: 10.1053/j.ajkd.2015.12.020. Epub 2016 Feb 2. Am J Kidney Dis. 2016. PMID: 26846333 Clinical Trial.
-
Oral roxadustat three times weekly in ESA-naïve and ESA-converted patients with anemia of chronic kidney disease on hemodialysis: Results from two phase 3 studies.Ther Apher Dial. 2020 Dec;24(6):628-641. doi: 10.1111/1744-9987.13468. Epub 2020 Feb 5. Ther Apher Dial. 2020. PMID: 31891449 Free PMC article. Clinical Trial.
-
Roxadustat (FG-4592) treatment for anemia in dialysis-dependent (DD) and not dialysis-dependent (NDD) chronic kidney disease patients: A systematic review and meta-analysis.Pharmacol Res. 2020 May;155:104747. doi: 10.1016/j.phrs.2020.104747. Epub 2020 Mar 17. Pharmacol Res. 2020. PMID: 32171893
-
Roxadustat for the treatment of anemia in patients with chronic kidney diseases: a meta-analysis.Aging (Albany NY). 2021 Jun 11;13(13):17914-17929. doi: 10.18632/aging.203143. Epub 2021 Jun 11. Aging (Albany NY). 2021. PMID: 34115611 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
