Prurigo nodularis: new insights into pathogenesis and novel therapeutics
- PMID: 38345154
- PMCID: PMC11099982
- DOI: 10.1093/bjd/ljae052
Prurigo nodularis: new insights into pathogenesis and novel therapeutics
Abstract
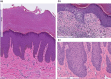
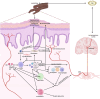
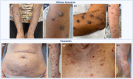
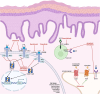
Prurigo nodularis (PN) is an inflammatory skin condition characterized by intensely pruritic nodules on the skin. Patients with PN suffer from an intractable itch-scratch cycle leading to impaired sleep, psychosocial distress and a significant disruption in quality of life. The pathogenesis of PN is associated with immune and neural dysregulation, mediated by inflammatory cytokines [such as interleukin (IL)-4, -13, -17, -22 and -31] and neuropeptides (such as substance P and calcitonin gene-related peptide). There is a role for type 2 inflammation in PN in addition to T-helper (Th)17 and Th22-mediated inflammation. The neuroimmune feedback loop in PN involves neuropeptides released from nerve fibres that cause vasodilation and further recruitment of inflammatory cells. Inflammatory cells, particularly mast cells and eosinophils, degranulate and release neurotoxins, as well as nerve growth factor, which may contribute to the neuronal hyperplasia seen in the dermis of patients with PN and neural sensitization. Recent studies have also indicated underlying genetic susceptibility to PN in addition to environmental factors, the existence of various disease endotypes centred around degrees of type 2 inflammation or underlying myelopathy or spinal disc disease, and significant race and ethnicity-based differences, with African Americans having densely fibrotic skin lesions. Dupilumab became the first US Food and Drug Administration-approved therapeutic for PN, and there are several other agents currently in development. The anti-IL-31 receptor A inhibitor nemolizumab is in late-stage development with positive phase III data reported. In addition, the oral Janus kinase (JAK) 1 inhibitors, abrocitinib and povorcitinib, are in phase II trials while a topical JAK1/2 inhibitor, ruxolitinib, is in phase III studies.
Plain language summary
Prurigo nodularis (PN) is a chronic skin condition featuring extremely itchy nodules on the skin of the legs, arms and trunk of the body. PN affects approximately 72 per 100 000 people and the severe itch associated with the condition can negatively impact a person’s sleep, work and social life. However, the cause of PN remains unclear. Current understanding of PN is based on imbalances in the immune system leading to widespread inflammation as well as dysregulation of the nerves in the skin. Immune molecules released from T cells [such as interleukin (IL)-4, -13, -31, -17, -22 and -31] increase systemic inflammation and are elevated in people with PN. Activated inflammatory cells (such as mast cells or eosinophils) may also release factors that promote inflammation, itch and neural changes within the skin. Neural dysregulation in PN features a lower density of itch-sensing nerve fibres in the epidermis (upper layer of the skin) and a higher density of itch-sensing nerve fibres in the dermis (lower layer of the skin). Because the pathogenesis of PN is not fully understood, the therapies available for PN have had limited success in reducing itch and nodules. The only drug currently approved for PN in the USA and Europe is dupilumab, an IL-4Rα inhibitor that blocks signalling through IL-4 and IL-13, which is undergoing post-marketing surveillance. Other new drugs are being assessed in various phases of clinical trials, including nemolizumab, vixarelimab, barzolvolimab, ruxolitinib, abrocitinib, povorcitinib and nalbuphine.
© The Author(s) 2024. Published by Oxford University Press on behalf of British Association of Dermatologists. All rights reserved. For commercial re-use, please contact reprints@oup.com for reprints and translation rights for reprints. All other permissions can be obtained through our RightsLink service via the Permissions link on the article page on our site—for further information please contact journals.permissions@oup.com.
Conflict of interest statement
Conflicts of interest S.G.K. is an advisory board member/consultant for AbbVie, Amgen, Arcutis Biotherapeutics, Aslan Pharmaceuticals, Cara Therapeutics, Castle Biosciences, Celldex Therapeutics, Galderma, Genzada Pharmaceuticals, Incyte Corporation, Johnson & Johnson, LEO Pharma, Novartis Pharmaceuticals Corporation, Pfizer, Regeneron Pharmaceuticals and Sanofi, and has served as an investigator for Galderma, Incyte, Pfizer and Sanofi. Specifically to prurigo nodularis, S.G.K. has received honoraria for serving as a consultant and advisory board member for the following relevant conflicts of interest: Sanofi-Regeneron, which has developed dupilumab for the treatment of prurigo nodularis (PN); Galderma, which is developing nemolizumab for the treatment of PN; Incyte, which is developing topical ruxolitinib and povorcitinib for the treatment of PN; and Celldex therapeutics, which is developing barzolvolimab for PN.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

