Neutrophil Granulopoiesis Optimized Through Ex Vivo Expansion of Hematopoietic Progenitors in Engineered 3D Gelatin Methacrylate Hydrogels
- PMID: 38345178
- PMCID: PMC11144100
- DOI: 10.1002/adhm.202301966
Neutrophil Granulopoiesis Optimized Through Ex Vivo Expansion of Hematopoietic Progenitors in Engineered 3D Gelatin Methacrylate Hydrogels
Abstract
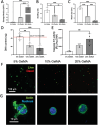
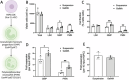
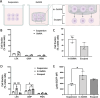
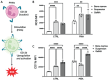
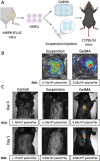
Neutrophils are the first line of defense of the innate immune system. In response to methicillin-resistant Staphylococcus aureus infection in the skin, hematopoietic stem, and progenitor cells (HSPCs) traffic to wounds and undergo extramedullary granulopoiesis, producing neutrophils necessary to resolve the infection. This prompted the engineering of a gelatin methacrylate (GelMA) hydrogel that encapsulates HSPCs within a matrix amenable to subcutaneous delivery. The authors study the influence of hydrogel mechanical properties to produce an artificial niche for granulocyte-monocyte progenitors (GMPs) to efficiently expand into functional neutrophils that can populate infected tissue. Lin-cKIT+ HSPCs, harvested from fluorescent neutrophil reporter mice, are encapsulated in GelMA hydrogels of varying polymer concentration and UV-crosslinked to produce HSPC-laden gels of specific stiffness and mesh sizes. Softer 5% GelMA gels yield the most viable progenitors and effective cell-matrix interactions. Compared to suspension culture, 5% GelMA results in a twofold expansion of mature neutrophils that retain antimicrobial functions including degranulation, phagocytosis, and ROS production. When implanted dermally in C57BL/6J mice, luciferase-expressing neutrophils expanded in GelMA hydrogels are visualized at the site of implantation for over 5 days. They demonstrate the potential of GelMA hydrogels for delivering HSPCs directly to the site of skin infection to promote local granulopoiesis.
Keywords: extramedullary granulopoiesis; gelatin methacrylate (GelMA); hematopoietic stem and progenitor cells (HSPCs); neutrophils.
© 2024 The Authors. Advanced Healthcare Materials published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Molecular interactions and forces of adhesion between single human neural stem cells and gelatin methacrylate hydrogels of varying stiffness.Acta Biomater. 2020 Apr 1;106:156-169. doi: 10.1016/j.actbio.2020.02.023. Epub 2020 Feb 18. Acta Biomater. 2020. PMID: 32084598
-
Towards Mimicking the Fetal Liver Niche: The Influence of Elasticity and Oxygen Tension on Hematopoietic Stem/Progenitor Cells Cultured in 3D Fibrin Hydrogels.Int J Mol Sci. 2020 Sep 2;21(17):6367. doi: 10.3390/ijms21176367. Int J Mol Sci. 2020. PMID: 32887387 Free PMC article.
-
Soluble Signals and Remodeling in a Synthetic Gelatin-Based Hematopoietic Stem Cell Niche.Adv Healthc Mater. 2019 Oct;8(20):e1900751. doi: 10.1002/adhm.201900751. Epub 2019 Sep 18. Adv Healthc Mater. 2019. PMID: 31532901 Free PMC article.
-
Gelatin Methacrylate (GelMA)-Based Hydrogels for Cell Transplantation: an Effective Strategy for Tissue Engineering.Stem Cell Rev Rep. 2019 Oct;15(5):664-679. doi: 10.1007/s12015-019-09893-4. Stem Cell Rev Rep. 2019. PMID: 31154619 Review.
-
Recent advances on gelatin methacrylate hydrogels with controlled microstructures for tissue engineering.Int J Biol Macromol. 2022 Nov 30;221:91-107. doi: 10.1016/j.ijbiomac.2022.08.171. Epub 2022 Aug 31. Int J Biol Macromol. 2022. PMID: 36057299 Review.
References
-
- Hirai H., Zhang P., Dayaram T., Hetherington C. J., Mizuno S., Imanishi J., Akashi K., Tenen D. G., Nat. Immunol. 2006, 7, 732. - PubMed
-
- Nauseef W. M., Borregaard N., Nat. Immunol. 2014, 15, 602. - PubMed
-
- Hämäläinen S., Kuittinen T., Matinlauri I., Nousiainen T., Koivula I., Jantunen E., Leuk Lymphoma 2008, 49, 495. - PubMed

