Risk of multiple sclerosis in patients with psoriasis receiving anti-IL-17 agents: A case-based review
- PMID: 38345289
- PMCID: PMC11484131
- DOI: 10.1111/1346-8138.17143
Risk of multiple sclerosis in patients with psoriasis receiving anti-IL-17 agents: A case-based review
Abstract
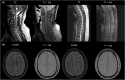
Biologics approved for psoriasis exhibit favorable safety profiles, and serious adverse events have rarely been reported. In this report, we present the case of a patient treated with ixekizumab, an anti-interleukin (IL)-17 agent, who 8 months later developed multiple sclerosis (MS). We also review the available literature regarding the use of anti-IL-17 agents in the context of psoriasis and pre-existing or new-onset demyelination. Eight case reports were evaluated as relevant and are presented in our report. In most of the cases secukinumab or ixekizumab administration adequately controlled both skin and pre-existing neurological clinical manifestations. However, there has been a report of MS exacerbation under secukinumab treatment and the occurrence of myelitis in a patient receiving ixekizumab. While the anti-IL-17-biologic-mediated induction of inflammatory events in the central nervous system has not been proven and a causal relationship is lacking, such a probability should be considered in extremely rare cases.
Keywords: MS; anti‐IL‐17; demyelination; psoriasis.
© 2024 The Authors. The Journal of Dermatology published by John Wiley & Sons Australia, Ltd on behalf of Japanese Dermatological Association.
Conflict of interest statement
Dimitrios P. Bogdanos declares lecture honoraria (2020–2023) from Abbvie Pharmaceuticals SA, Novartis, Menarini Hellas, Boehringer Ingelheim, Genesis Pharma, Pharmaserv‐Lilly, Fresenius Kabi, Euroimmun, Werfen, Sobi; congress travel and accommodation support from Abbvie, Novartis, Hospital Line, Pfizer, Elpen, Aenorasis Hellas, Pharmaserv‐Lilly, Sobi. Efterpi Zafiriou declares consulting fees from Abbvie, Genesis Pharma, Leo, Novartis, Jannsen, Pharmaserv Lilly, Pfizer, Sanofi, UCB, payment or honoraria: Abbvie, Genesis Pharma, Leo, Novartis, Jannsen, Pharmaserv Lilly, Pfizer, Sanofi, UCB, support for attending meetings: Abbvie, Genesis Pharma, Novartis, Jannsen, Pharmaserv Lilly, Sanofi, UCB. Sotirios G. Tsiogkas, Vaia Tsimourtou, Kleoniki Chaidaki, Efthymios Dardiotis, and Angeliki Victoria Roussaki‐Schulze declare no conflicts of interest.
Figures
References
-
- Gratton D, Szapary P, Goyal K, Fakharzadeh S, Germain V, Saltiel P. Reversible posterior leukoencephalopathy syndrome in a patient treated with ustekinumab: case report and review of the literature. Arch Dermatol. 2011;147:1197–1202. - PubMed
-
- Badat Y, Meissner WG, Laharie D. Demyelination in a patient receiving ustekinumab for refractory Crohn's disease. J Crohns Colitis. 2014;8:1138–1139. - PubMed
-
- Hamadah I, Chisti MA. Axonal sensorimotor polyneuropathy after starting guselkumab. J Dermatolog Treat. 2022;33:2371–2372. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical