Discrete Acyltransferases and Thioesterases in Iso-Migrastatin and Lactimidomycin Biosynthesis
- PMID: 38345531
- PMCID: PMC11623918
- DOI: 10.1021/acs.biochem.3c00672
Discrete Acyltransferases and Thioesterases in Iso-Migrastatin and Lactimidomycin Biosynthesis
Abstract
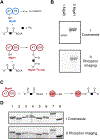
Iso-Migrastatin (iso-MGS) and lactimidomycin (LTM) are glutarimide-containing polyketide natural products (NPs) that are biosynthesized by homologous acyltransferase (AT)-less type I polyketide synthase (PKS) assembly lines. The biological activities of iso-MGS and LTM have inspired numerous efforts to generate analogues via genetic manipulation of their biosynthetic machinery in both native producers and model heterologous hosts. A detailed understanding of the MGS and LTM AT-less type I PKSs would serve to inspire future engineering efforts while advancing the fundamental knowledge of AT-less type I PKS enzymology. The mgs and ltm biosynthetic gene clusters (BGCs) encode for two discrete ATs of the architecture AT-enoylreductase (AT-ER) and AT-type II thioesterase (AT-TE). Herein, we report the functional characterization of the mgsB and ltmB and the mgsH and ltmH gene products, revealing that MgsB and LtmB function as type II thioesterases (TEs) and MgsH and LtmH are the dedicated trans-ATs for the MGS and LTM AT-less type I PKSs. In vivo and in vitro experiments demonstrated that MgsB was devoid of any AT activity, despite the presence of the conserved catalytic triad of canonical ATs. Cross-complementation experiments demonstrated that MgsH and LtmH are functionally interchangeable between the MGS and LTM AT-less type I PKSs. This work sets the stage for future mechanistic studies of AT-less type I PKSs and efforts to engineer the MGS and LTM AT-less type I PKS assembly lines for novel glutarimide-containing polyketides.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


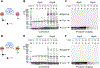

References
-
- Nakae K; Yoshimoto Y; Sawa T; Homma Y; Hamada M; Takeuchi T; Imoto M Migrastatin, a New Inhibitor of Tumor Cell Migration from Streptomyces sp. MK929–43F1. Taxonomy, Fermentation, Isolation and Biological Activities. J. Antibiot. 2000, 53, 1130–1136. - PubMed
-
- Sugawara K; Nishiyama Y; Toda S; Komiyama N; Hatori M; Moriyama T; Sawada Y; Kamei H; Konishi M; Oki T Lactimidomycin, a New Glutarimide Group Antibiotic. Production, Isolation, Structure and Biological Activity. J. Antibiot. 1992, 45, 1433–1441. - PubMed
-
- Gaul C; Njardarson JT; Shan D; Dorn DC; Wu KD; Tong WP; Huang XY; Moore MA; Danishefsky SJ The Migrastatin Family: Discovery of Potent Cell Migration Inhibitors by Chemical Synthesis. J. Am. Chem. Soc. 2004, 126, 11326–11337. - PubMed
-
- Karwowski JP; Jackson M; Sunga G; Sheldon P; Poddig JB; Kohl WL; Kadam S Dorrigocins: Novel Antifungal Antibiotics that Change the Morphology of Ras-Transformed NIH/3T3 Cells to that of Normal Cells. I. Taxonomy of the Producing Organism, Fermentation and Biological Activity. J. Antibiot. 1994, 47, 862–869. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

