Real-World Use and Effectiveness Outcomes in Patients with Rheumatoid Arthritis Treated with Upadacitinib: An Analysis from the CorEvitas Registry
- PMID: 38345715
- PMCID: PMC10920593
- DOI: 10.1007/s40744-024-00639-4
Real-World Use and Effectiveness Outcomes in Patients with Rheumatoid Arthritis Treated with Upadacitinib: An Analysis from the CorEvitas Registry
Abstract
Introduction: Data assessing longer-term real-world effectiveness and treatment patterns with upadacitinib (UPA), a Janus kinase inhibitor, in rheumatoid arthritis (RA) are lacking. We assessed improvement in clinical and patient-reported outcomes and treatment patterns for up to 12 months among adult patients with RA initiating UPA.
Methods: Data were collected from the CorEvitas® RA Registry (08/2019-04/2022). Eligible patients had moderate to severe RA (Clinical Disease Activity Index [CDAI] > 10) and follow-up visits at 6 or 12 months after UPA initiation. Outcomes were mean change from baseline, percentage achieving minimal clinically important differences (MCID) in clinical and patient-reported outcomes, and disease activity at follow-up. We evaluated clinical outcomes and therapy changes among patients with tumor necrosis factor inhibitor (TNFi) experience and among those receiving UPA as first-line therapy, as well as those receiving UPA as monotherapy versus as part of combination therapy. We further evaluated whether outcomes were similar among those that remained on therapy.
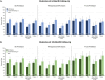
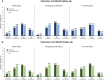
Results: Patients treated with UPA (6-month cohort, N = 469; 12-month cohort, N = 263) had statistically significant improvements (p < 0.001) in mean CDAI, tender/swollen joint counts, pain, and fatigue at follow-up. At 12 months, 46.0% achieved MCID in CDAI and 40.0% achieved low disease activity/remission. Overall, 43.0% discontinued UPA at 12 months; of those receiving combination treatment (N = 90) with conventional therapies and UPA, 42.2% (N = 38) discontinued conventional therapy. Findings were similar in the 6-month cohort and among subgroups. Changes from baseline and proportions of patients achieving MCID or clinical outcomes tended to be numerically lower among patients with TNFi experience and numerically higher among those receiving UPA as first-line therapy.
Conclusions: UPA initiation was associated with improvements in clinical and patient-reported outcomes, with meaningful clinical improvements regardless of prior TNFi experience, line of therapy, or concomitant use of conventional therapies. Further research is needed to better understand sustained response of UPA over longer treatment periods.
Keywords: Patient-reported outcomes; Real-world evidence; Registry; Rheumatoid arthritis; Upadacitinib.
© 2024. The Author(s).
Conflict of interest statement
Joshua F. Baker has received consulting fees from CorEvitas and Cumberland Pharma and has received funding from Horizon Pharma. Denise Bennett, Miao Yu, Yolanda Munoz Maldonado, and Robert R. McLean are employees of CorEvitas (previously Corrona, LLC). Mira Ali and Patrick Zueger are employees of AbbVie Inc. and may own stock.
Figures
Similar articles
-
Real-World Persistence and Effectiveness of Upadacitinib versus Other Janus Kinase Inhibitors and Tumor Necrosis Factor Inhibitors in Australian Patients with Rheumatoid Arthritis.Rheumatol Ther. 2025 Feb;12(1):173-202. doi: 10.1007/s40744-024-00736-4. Epub 2025 Jan 6. Rheumatol Ther. 2025. PMID: 39757285 Free PMC article.
-
A Real-World Effectiveness Study Using a Mobile Application to Evaluate Early Outcomes with Upadacitinib in Rheumatoid Arthritis.Rheumatol Ther. 2023 Dec;10(6):1519-1533. doi: 10.1007/s40744-023-00594-6. Epub 2023 Sep 20. Rheumatol Ther. 2023. PMID: 37728861 Free PMC article.
-
Treatment patterns and clinical outcomes in patients with rheumatoid arthritis initiating etanercept, adalimumab, or Janus kinase inhibitor as first-line therapy: results from the real-world CorEvitas RA Registry.Arthritis Res Ther. 2023 Sep 9;25(1):166. doi: 10.1186/s13075-023-03120-9. Arthritis Res Ther. 2023. PMID: 37689684 Free PMC article.
-
A Real-World Comparison of Clinical Effectiveness in Patients with Rheumatoid Arthritis Treated with Upadacitinib, Tumor Necrosis Factor Inhibitors, and Other Advanced Therapies After Switching from an Initial Tumor Necrosis Factor Inhibitor.Adv Ther. 2024 Sep;41(9):3706-3721. doi: 10.1007/s12325-024-02948-0. Epub 2024 Aug 7. Adv Ther. 2024. PMID: 39110310 Free PMC article.
-
Sarilumab for Previously-Treated Moderate or Severe Rheumatoid Arthritis: An Evidence Review Group Perspective of a NICE Single Technology Appraisal.Pharmacoeconomics. 2018 Dec;36(12):1427-1437. doi: 10.1007/s40273-018-0677-7. Pharmacoeconomics. 2018. PMID: 29882210 Review.
Cited by
-
Achievement of treatment targets and maintenance of response with upadacitinib in patients with moderate-to-severe rheumatoid arthritis in real-world practice: 1-year outcomes from the UPHOLD observational study.Arthritis Res Ther. 2025 Apr 10;27(1):84. doi: 10.1186/s13075-025-03528-5. Arthritis Res Ther. 2025. PMID: 40211417 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

