HS-GC-MS analysis of volatile organic compounds after hyperoxia-induced oxidative stress: a validation study
- PMID: 38345723
- PMCID: PMC10861410
- DOI: 10.1186/s40635-024-00600-3
HS-GC-MS analysis of volatile organic compounds after hyperoxia-induced oxidative stress: a validation study
Abstract
Background: Exhaled volatile organic compounds (VOCs), particularly hydrocarbons from oxidative stress-induced lipid peroxidation, are associated with hyperoxia exposure. However, important heterogeneity amongst identified VOCs and concerns about their precise pathophysiological origins warrant translational studies assessing their validity as a marker of hyperoxia-induced oxidative stress. Therefore, this study sought to examine changes in VOCs previously associated with the oxidative stress response in hyperoxia-exposed lung epithelial cells.
Methods: A549 alveolar epithelial cells were exposed to hyperoxia for 24 h, or to room air as normoxia controls, or hydrogen peroxide as oxidative-stress positive controls. VOCs were sampled from the headspace, analysed by gas chromatography coupled with mass spectrometry and compared by targeted and untargeted analyses. A secondary analysis of breath samples from a large cohort of critically ill adult patients assessed the association of identified VOCs with clinical oxygen exposure.
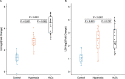
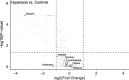
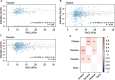
Results: Following cellular hyperoxia exposure, none of the targeted VOCs, previously proposed as breath markers of oxidative stress, were increased, and decane was significantly decreased. Untargeted analysis did not reveal novel identifiable hyperoxia-associated VOCs. Within the clinical cohort, three previously proposed breath markers of oxidative stress, hexane, octane, and decane had no real diagnostic value in discriminating patients exposed to hyperoxia.
Conclusions: Hyperoxia exposure of alveolar epithelial cells did not result in an increase in identifiable VOCs, whilst VOCs previously linked to oxidative stress were not associated with oxygen exposure in a cohort of critically ill patients. These findings suggest that the pathophysiological origin of previously proposed breath markers of oxidative stress is more complex than just oxidative stress from hyperoxia at the lung epithelial cellular level.
Keywords: Headspace gas chromatography–mass spectrometry; Hyperoxia; Intensive care unit; Mechanical ventilation; Oxidative stress; Volatile organic compounds.
© 2024. The Author(s).
Conflict of interest statement
PB reports grants from Amsterdam UMC (Innovation Impulse grant), Vertex (Vertex Innovation Award), Stichting Astma Bestrijding (SAB grant), Boehringer Ingelheim Grant, Eurostars (Public–Private Partnership grant), Horizon Europe Framework Programme (HORIZON grant) outside the submitted work.
LDJ reports grants from the Dutch lung foundation (Young investigator grant), grants from the Dutch lung foundation and Health Holland (Public–Private Partnership grant), grants from the Dutch lung foundation (Dirkje Postma Award), grants from IMI COVID19 initiative, grants from Amsterdam UMC fellowship, grants from ZonMW (VIDI) outside the submitted work. He has also served in advisory capacity for Sobi NL, Impentri, Novartis, AstraZeneca, CSL Behring and Scailyte with money paid to his institution.
All other authors have no competing interests to disclose.
Figures


References
-
- Clark JM, Lambertsen CJ. Pulmonary oxygen toxicity: a review. Pharmacol Rev. 1971;23(2):37–133. - PubMed
-
- Asfar P, Schortgen F, Boisrame-Helms J, Charpentier J, Guerot E, Megarbane B, et al. Hyperoxia and hypertonic saline in patients with septic shock (HYPERS2S): a two-by-two factorial, multicentre, randomised, clinical trial. Lancet Respir Med. 2017;5(3):180–190. doi: 10.1016/S2213-2600(17)30046-2. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

