Outcomes of Various Classes of Oral Antidiabetic Drugs on Nonalcoholic Fatty Liver Disease
- PMID: 38345802
- PMCID: PMC10862270
- DOI: 10.1001/jamainternmed.2023.8029
Outcomes of Various Classes of Oral Antidiabetic Drugs on Nonalcoholic Fatty Liver Disease
Abstract
Importance: Several oral antidiabetic drug (OAD) classes can potentially improve patient outcomes in nonalcoholic fatty liver disease (NAFLD) to varying degrees, but clinical data on which class is favored are lacking.
Objective: To investigate which OAD is associated with the best patient outcomes in NAFLD and type 2 diabetes (T2D).
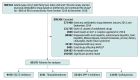
Design, setting, and participants: This retrospective nonrandomized interventional cohort study used the National Health Information Database, which provided population-level data for Korea. This study involved patients with T2D and concomitant NAFLD.
Exposures: Receiving either sodium-glucose cotransporter 2 (SGLT2) inhibitors, thiazolidinediones, dipeptidyl peptidase-4 (DPP-4) inhibitors, or sulfonylureas, each combined with metformin for 80% or more of 90 consecutive days.
Main outcomes and measures: The main outcomes were NAFLD regression assessed by the fatty liver index and composite liver-related outcome (defined as liver-related hospitalization, liver-related mortality, liver transplant, and hepatocellular carcinoma) using the Fine-Gray model regarding competing risks.
Results: In total, 80 178 patients (mean [SD] age, 58.5 [11.9] years; 43 007 [53.6%] male) were followed up for 219 941 person-years, with 4102 patients experiencing NAFLD regression. When compared with sulfonylureas, SGLT2 inhibitors (adjusted subdistribution hazard ratio [ASHR], 1.99 [95% CI, 1.75-2.27]), thiazolidinediones (ASHR, 1.70 [95% CI, 1.41-2.05]), and DPP-4 inhibitors (ASHR, 1.45 [95% CI, 1.31-1.59]) were associated with NAFLD regression. SGLT2 inhibitors were associated with a higher likelihood of NAFLD regression when compared with thiazolidinediones (ASHR, 1.40 [95% CI, 1.12-1.75]) and DPP-4 inhibitors (ASHR, 1.45 [95% CI, 1.30-1.62]). Only SGLT2 inhibitors (ASHR, 0.37 [95% CI, 0.17-0.82]), not thiazolidinediones or DPP-4 inhibitors, were significantly associated with lower incidence rates of adverse liver-related outcomes when compared with sulfonylureas.
Conclusions and relevance: The results of this cohort study suggest that physicians may lean towards prescribing SGLT2 inhibitors as the preferred OAD for individuals with NAFLD and T2D, considering their potential benefits in NAFLD regression and lower incidences of adverse liver-related outcomes. This observational study should prompt future research to determine whether prescribing practices might merit reexamination.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

