Cell contacts and pericellular matrix in the Xenopus gastrula chordamesoderm
- PMID: 38346069
- PMCID: PMC10861091
- DOI: 10.1371/journal.pone.0297420
Cell contacts and pericellular matrix in the Xenopus gastrula chordamesoderm
Abstract
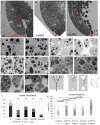
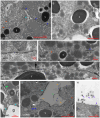
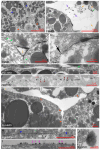
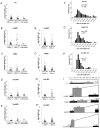
Convergent extension of the chordamesoderm is the best-examined gastrulation movement in Xenopus. Here we study general features of cell-cell contacts in this tissue by combining depletion of adhesion factors C-cadherin, Syndecan-4, fibronectin, and hyaluronic acid, the analysis of respective contact width spectra and contact angles, and La3+ staining of the pericellular matrix. We provide evidence that like in other gastrula tissues, cell-cell adhesion in the chordamesoderm is largely mediated by different types of pericellular matrix. Specific glycocalyx structures previously identified in Xenopus gastrula tissues are absent in chordamesoderm but other contact types like 10-20 nm wide La3+ stained structures are present instead. Knockdown of any of the adhesion factors reduces the abundance of cell contacts but not the average relative adhesiveness of the remaining ones: a decrease of adhesiveness at low contact widths is compensated by an increase of contact widths and an increase of adhesiveness proportional to width. From the adhesiveness-width relationship, we derive a model of chordamesoderm cell adhesion that involves the interdigitation of distinct pericellular matrix units. Quantitative description of pericellular matrix deployment suggests that reduced contact abundance upon adhesion factor depletion is correlated with excessive accumulation of matrix material in non-adhesive gaps and the loss of some contact types.
Copyright: © 2024 Luu et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



Similar articles
-
Cell-cell contact landscapes in Xenopus gastrula tissues.Proc Natl Acad Sci U S A. 2021 Sep 28;118(39):e2107953118. doi: 10.1073/pnas.2107953118. Proc Natl Acad Sci U S A. 2021. PMID: 34544871 Free PMC article.
-
Cadherin function during Xenopus gastrulation.Subcell Biochem. 2012;60:301-20. doi: 10.1007/978-94-007-4186-7_13. Subcell Biochem. 2012. PMID: 22674077 Review.
-
Ultrastructural study of contacts between cells of the dorsal ectoderm and chordamesoderm during gastrulation in Xenopus laevis.Acta Anat (Basel). 1988;133(1):5-9. doi: 10.1159/000146605. Acta Anat (Basel). 1988. PMID: 2463727
-
Induction of notochord cell intercalation behavior and differentiation by progressive signals in the gastrula of Xenopus laevis.Development. 1995 Oct;121(10):3311-21. doi: 10.1242/dev.121.10.3311. Development. 1995. PMID: 7588065
-
Cell adhesion in amphibian gastrulation.Int Rev Cell Mol Biol. 2009;278:215-75. doi: 10.1016/S1937-6448(09)78005-0. Int Rev Cell Mol Biol. 2009. PMID: 19815180 Review.
References
MeSH terms
LinkOut - more resources
Full Text Sources

