Intracellular C3 protects β-cells from IL-1β-driven cytotoxicity via interaction with Fyn-related kinase
- PMID: 38346191
- PMCID: PMC10895342
- DOI: 10.1073/pnas.2312621121
Intracellular C3 protects β-cells from IL-1β-driven cytotoxicity via interaction with Fyn-related kinase
Abstract
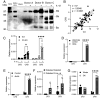
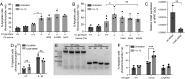
One of the hallmarks of type 1 but also type 2 diabetes is pancreatic islet inflammation, associated with altered pancreatic islet function and structure, if unresolved. IL-1β is a proinflammatory cytokine which detrimentally affects β-cell function. In the course of diabetes, complement components, including the central complement protein C3, are deregulated. Previously, we reported high C3 expression in human pancreatic islets, with upregulation after IL-1β treatment. In the current investigation, using primary human and rodent material and CRISPR/Cas9 gene-edited β-cells deficient in C3, or producing only cytosolic C3 from a noncanonical in-frame start codon, we report a protective effect of C3 against IL-1β-induced β-cell death, that is attributed to the cytosolic fraction of C3. Further investigation revealed that intracellular C3 alleviates IL-1β-induced β-cell death, by interaction with and inhibition of Fyn-related kinase (FRK), which is involved in the response of β-cells to cytokines. Furthermore, these data were supported by increased β-cell death in vivo in a β-cell-specific C3 knockout mouse. Our data indicate that a functional, cytoprotective association exists between FRK and cytosolic C3.
Keywords: C3; FRK; IL-1β; diabetes; β-cell.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures





References
-
- Donath M. Y., Shoelson S. E., Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 11, 98–107 (2011). - PubMed
-
- Ahlqvist E., et al. , Novel subgroups of adult-onset diabetes and their association with outcomes: A data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol. 6, 361–369 (2018). - PubMed
-
- Mandrup-Poulsen T., Pickersgill L., Donath M. Y., Blockade of interleukin 1 in type 1 diabetes mellitus. Nat. Rev. Endocrinol. 6, 158–166 (2010). - PubMed
-
- Larsen C. M., et al. , Interleukin-1 receptor antagonist-treatment of patients with type 2 diabetes. Ugeskr. Laeger 169, 3868–3871 (2007). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

