N-Substituted Bicyclic Carbamoyl Pyridones: Integrase Strand Transfer Inhibitors that Potently Inhibit Drug-Resistant HIV-1 Integrase Mutants
- PMID: 38346249
- PMCID: PMC10928719
- DOI: 10.1021/acsinfecdis.3c00525
N-Substituted Bicyclic Carbamoyl Pyridones: Integrase Strand Transfer Inhibitors that Potently Inhibit Drug-Resistant HIV-1 Integrase Mutants
Abstract
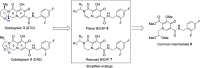
HIV-1 integrase (IN) is an important molecular target for the development of anti-AIDS drugs. A recently FDA-approved second-generation integrase strand transfer inhibitor (INSTI) cabotegravir (CAB, 2021) is being marketed for use in long-duration antiviral formulations. However, missed doses during extended therapy can potentially result in persistent low levels of CAB that could select for resistant mutant forms of IN, leading to virological failure. We report a series of N-substituted bicyclic carbamoyl pyridones (BiCAPs) that are simplified analogs of CAB. Several of these potently inhibit wild-type HIV-1 in single-round infection assays in cultured cells and retain high inhibitory potencies against a panel of viral constructs carrying resistant mutant forms of IN. Our lead compound, 7c, proved to be more potent than CAB against the therapeutically important resistant double mutants E138K/Q148K (>12-fold relative to CAB) and G140S/Q148R (>36-fold relative to CAB). A significant number of the BiCAPs also potently inhibit the drug-resistant IN mutant R263K, which has proven to be problematic for the FDA-approved second-generation INSTIs.
Keywords: HIV-1; INSTI; INSTI-resistance; bicyclic carbamoyl pyridone (BiCAP); cabotegravir; one-pot synthesis; resistant mutants.
Conflict of interest statement
The authors declare the following competing financial interest(s): aspects of the reported research may be covered in pending patent applications.
Figures


Similar articles
-
Antiviral Activity of Bictegravir and Cabotegravir against Integrase Inhibitor-Resistant SIVmac239 and HIV-1.Antimicrob Agents Chemother. 2017 Nov 22;61(12):e01695-17. doi: 10.1128/AAC.01695-17. Print 2017 Dec. Antimicrob Agents Chemother. 2017. PMID: 28923862 Free PMC article.
-
Efficacies of Cabotegravir and Bictegravir against drug-resistant HIV-1 integrase mutants.Retrovirology. 2018 May 16;15(1):37. doi: 10.1186/s12977-018-0420-7. Retrovirology. 2018. PMID: 29769116 Free PMC article.
-
Selective resistance profiles emerging in patient-derived clinical isolates with cabotegravir, bictegravir, dolutegravir, and elvitegravir.Retrovirology. 2018 Aug 17;15(1):56. doi: 10.1186/s12977-018-0440-3. Retrovirology. 2018. PMID: 30119633 Free PMC article.
-
Different Pathways Conferring Integrase Strand-Transfer Inhibitors Resistance.Viruses. 2022 Nov 22;14(12):2591. doi: 10.3390/v14122591. Viruses. 2022. PMID: 36560595 Free PMC article. Review.
-
HIV-1 integrase strand transfer inhibitors: a review of current drugs, recent advances and drug resistance.Int J Antimicrob Agents. 2021 May;57(5):106343. doi: 10.1016/j.ijantimicag.2021.106343. Epub 2021 Apr 11. Int J Antimicrob Agents. 2021. PMID: 33852932 Review.
References
-
- Johns B. A.; Svolto A. C. Advances in Two-metal Chelation Inhibitors of HIV Integrase. Expert Opin. Ther. Pat. 2008, 18, 1225–1237. 10.1517/13543776.18.11.1225. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

