Global guideline for the diagnosis and management of cryptococcosis: an initiative of the ECMM and ISHAM in cooperation with the ASM
- PMID: 38346436
- PMCID: PMC11526416
- DOI: 10.1016/S1473-3099(23)00731-4
Global guideline for the diagnosis and management of cryptococcosis: an initiative of the ECMM and ISHAM in cooperation with the ASM
Erratum in
-
Correction to Lancet Infect Dis 2024; published online Feb 9. https://doi.org/10.1016/S1473-3099(23)00731-4.Lancet Infect Dis. 2024 Aug;24(8):e485. doi: 10.1016/S1473-3099(24)00426-2. Epub 2024 Jun 27. Lancet Infect Dis. 2024. PMID: 38945148 No abstract available.
Abstract
Cryptococcosis is a major worldwide disseminated invasive fungal infection. Cryptococcosis, particularly in its most lethal manifestation of cryptococcal meningitis, accounts for substantial mortality and morbidity. The breadth of the clinical cryptococcosis syndromes, the different patient types at-risk and affected, and the vastly disparate resource settings where clinicians practice pose a complex array of challenges. Expert contributors from diverse regions of the world have collated data, reviewed the evidence, and provided insightful guideline recommendations for health practitioners across the globe. This guideline offers updated practical guidance and implementable recommendations on the clinical approaches, screening, diagnosis, management, and follow-up care of a patient with cryptococcosis and serves as a comprehensive synthesis of current evidence on cryptococcosis. This Review seeks to facilitate optimal clinical decision making on cryptococcosis and addresses the myriad of clinical complications by incorporating data from historical and contemporary clinical trials. This guideline is grounded on a set of core management principles, while acknowledging the practical challenges of antifungal access and resource limitations faced by many clinicians and patients. More than 70 societies internationally have endorsed the content, structure, evidence, recommendation, and pragmatic wisdom of this global cryptococcosis guideline to inform clinicians about the past, present, and future of care for a patient with cryptococcosis.
Copyright © 2024 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests AA reports grants from the Agence Nationale de la Recherche; serving as a consultant to Gilead Sciences; receiving speaking honoraria from Gilead Sciences and PR Edition; travel support from Gilead sciences and Pfizer; and patents with the Institut Pasteur. J-WA reports grants or contracts from WHO (fungal priority pathogens list) and receipt of equipment and materials from the Westmead Hospital Foundation. JB reports support from the Australian National Health and Medical Research Council and receipt of honoraria from Gilead. TAB reports a personal research fellowship from Gilead Sciences; investigator-led research grant from Pfizer; lecture honoraria and participation in advisory boards for Gilead Sciences, Mundipharma, and Pfizer; and participation in the Trial Steering Committee for a phase 2 trial of inhaled opelconazole (Pulmocide). FC reports speaker honoraria from, and being part of, an advisory board for Pfizer and United Medical. CCC reports receipt of an Early Career Fellowship from the Australian National Health and Medical Research Foundation, receipt of a speaker travel support for IDweek 2024, being a principal investigator in an early phase clinical trial unit, and was a recipient of the Australian National Health and Medical Research Council Early Career Fellowship (APP 1092160). MC reports grants from Cidara, F2G, Pfizer, and Janssen; receipt of honoraria from Pfizerm MSD and Gilead; and travel support from Pfizer. SCC reports untied educational grants from MSD Australia and F2G and is on the antifungal advisory boards of MSD Australia, Gilead Sciences, and F2G. OAC reports grants or contracts from BMBF, Cidara, EU-DG RTD (101037867), F2G, Gilead, MedPace, MSD, Mundipharma, Octapharma, Pfizer, and Scynexis; consulting fees from AbbVie, AiCuris, Biocon, Cidara, Gilead, IQVIA, Janssen, Matinas, MedPace, Menarini, Moderna, Molecular Partners, MSG-ERC, Noxxon, Octapharma, Pfizer, PSI, Scynexis, and Seres; honoraria for lectures from Abbott, AbbVie, Al-Jazeera Pharmaceuticals, Hikma, Gilead, Grupo Biotoscana/United Medical/Knight, MedScape, MedUpdate, Merck/MSD, Noscendo, Pfizer, Shionogi, and streamedup!; payment for expert testimony from Cidara; participation on a data safety monitoring board or advisory board from Boston Strategic Partners, Cidara, IQVIA, Janssen, MedPace, PSI, Pulmocide, Shionogi, and The Prime Meridian Group; a patent at the German Patent and Trade Mark Office (DE 10 2021 113 007·7); stocks from CoRe Consulting and EasyRadiology; other interests from Wiley; support from the German Federal Ministry of Research and Education; and funding by the Deutsche Forschungsgemeinschaft under Germany's Excellence Strategy (Cologne Cluster of Excellence on Cellular Stress Responses in Aging-associated Diseases, EXC 2030—390661388). J-PG reports speaker honoraria from Gilead, MundiPharma, and Pfizer. NPG reports grants from National Institutes of Health (USA), National Institute of Health and Care Research (UK), Medical Research Council (MRC; UK), Centers for Disease Control and Prevention (CDC; USA), and National Health Laboratory Service Research Trust (South Africa); participation in the ACACIA trial as part of the data safety monitoring board, project committee of DREAMM, project advisory committee for 5FC Crypto, and leadership roles in the Federation of Infectious Diseases Societies of Southern Africa. AHG reports grants from Gilead Sciences; personal fees from Amplyx, Astellas, Basilea, F2G, Gilead Sciences, Merck Sharp & Dohme, Mundipharma, Pfizer, and Scynexis; speaker honoraria from Gilead Sciences and MSD; and participation in an advisory board for Astellas, Mundipharma, Partner Therapeutics, and Pfizer. FH reports grants from Health Holland and European Society for Clinical Microbiology and Infectious Diseases; leadership roles as treasurer of the Netherlands Society for Medical Mycology, Chair of the Division Microbial Genomics of the Royal Netherlands Society for Microbiology, Vice-President International Society for Human and Animal Mycology (ISHAM); and receipt of evaluation kits from Bruker and Pathonostics. TSH reports receipt of an investigator award from Gilead Sciences, honoraria from Pfizer and Gilead Sciences, and participation in a data safety monitoring board or advisory board for Viamet and F2G. MH reports receipt of an European and Developing Countries Clinical Trials Partnership. JNJ reports support from the National Institute for Health Research; grants from European and Developing Countries Clinical Trials Partnership, joint global health trials (Wellcome Trust, MRC, and UK aid) and CDC; speaker fees from Gilead Sciences; participation on a data safety and monitoring board for the HARVEST, ARTIST, CASTLE, and ACACIA trials. GJ reports travel support to attend a meeting at ISHAM. NK was a speaker and advisor for Gilead Sciences, Merck/MSD, and Pfizer and a speaker for Astellas. MSL reports support from the Division of Intramural Research, National Institute of Allergy and Infectious Diseases (NIAID), and National Institutes of Health (NIH). OL reports receipt of consulting fees and honoraria from Gilead Science and patents with INSERM APHP. OMM reports travel support for ISHAM meeting in India and being the country ambassador for Kenya for ISHAM. BJM reports being chair of the Australia and New Zealand Paediatric Infectious Diseases Group. DBM reports leadership role in the Crypto Meningitis advocacy group. RO reports receiving research and educational grant funding from Gilead Sciences, CDC Atlanta, and Pfizer Specialties and travel support from the CDC foundation. PGP reports grants from Mayne, Astellas, Scynexis, and Cidara and receipt of consulting fees from F2G and Cidara. AKP reports speaker honoraria for Gilead Science, Pfizer India, and Intas pharmaceutical. JRP reports grants from NIH, Appili, and Sfunga; royalties from Up-To-Date; and participation on a data safety monitoring board or advisory board from Pulmocide, EFFECT trial, and IMPRINT trial. FQ-TF reports receipt of speaker honoraria from Pfizer and United Medical, travel support and laboratory diagnostic kits from IMMY, and leadership roles in Infocus Latin America. JS-G reports speaker honoraria from Gilead and Pfizer and is on an advisory committee for Pfizer. AS reports grants from Astellas and receiving consulting fees from Scynexis. RSp has received speaker honoraria from Pfizer and reports being chair of Young European Confederation of Medical Mycology. TT reports receipt of honoraria from Pfizer, MSD, Asahikasei pharma, and Sumitomo pharma. AW reports a grant from UK Research and Innovation; receipt of consultant fees from Gilead and MundiPharma; speaker fees from F2G and Gilead; and participation as a data safety monitoring board member for the RECOVERY trial. All declarations are outside the submitted work. All other authors declare no competing interests.
Figures
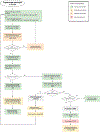

*(L-Amb 3–4 mg/kg/d + 5-FC 25 mg/kg 4x a day for 2 weeks) has not been compared to #(Single dose 10mg/kg L-Amb with 14d of 5-FC 25.kg 4x a day and Fluconazole 1200 mg daily). # has only been trialled in HIV-CM.
&Polyene includes Amphotericin B formulations such as conventional deoxycholate Amphotericin B (Amb-D), Liposomal Amphotericin (L-Amb), Amphotericin B lipid complex (ABLC).
^Amb-D: 1 mg/kg showed earlier fungicidal activity than 0.7 mg/kg but some institutions use the lower dose due to toxicity concerns.
@Fluconazole induction doses of up to 1200 mg daily have been trialled but caution is advised. Consider drug-drug interaction and liver toxicity.
Grading of recommendation and level of evidence in bolded red letters.
Recommendations by shading: green (Strong), yellow (Moderate), pink (marginal)

References
-
- (WHO) WHO. WHO fungal priority pathogens list to guide research, development and public health action.. Geneva.
-
- Brouwer AE, Rajanuwong A, Chierakul W, et al. Combination antifungal therapies for HIV-associated cryptococcal meningitis: a randomised trial. Lancet 2004; 363(9423): 1764–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

