Disease activity-guided dose optimization including discontinuation of TNF inhibitors in rheumatoid arthritis is effective for up to 10 years: an observational follow-up of the DRESS study
- PMID: 38346712
- PMCID: PMC11781578
- DOI: 10.1093/rheumatology/keae103
Disease activity-guided dose optimization including discontinuation of TNF inhibitors in rheumatoid arthritis is effective for up to 10 years: an observational follow-up of the DRESS study
Abstract
Objective: The objective of this study was to investigate the safety and effectiveness of disease activity-guided dose optimization of TNF inhibitors in RA over 10 years.
Methods: The study involved an observational long-term extension of a randomized study of participants who completed the 3-year extension of the DRESS-study. After the randomized phase (months 0-18), disease activity-guided dose optimization was allowed for all. The main outcomes were mean time-weighted DAS28-CRP; biologic and targeted synthetic DMARD (b/tsDMARD) use per year, as proportion of daily defined dose; proportion of patients reaching discontinuation; durability and effectiveness of subsequent dose reduction attempts; and radiographic progression between years 3 and 10 using the Sharp-van der Heijde score.
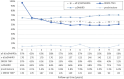
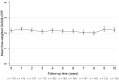
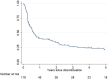
Results: A total of 170 patients were included, of whom 127 completed the 10-year follow-up. The mean disease activity remained low (DAS28-CRP 2.13, 95% CI 2.10-2.16), while the b/tsDMARD dose reduced from 97% at baseline (95% CI 96-99%, n = 170) to 56% at year 10 (95% CI 49-63%, n = 127). Of 161 participants with an optimization attempt, 119 (74%) reached discontinuation with a median duration of 7 months (interquartile range 3-33 months), and 25 participants never had to restart their b/tsDMARD (21%, 95% CI 14-29%). The mean dose reduction after dose optimization was 48% (n = 159) for the first optimization attempt, and 33% for a subsequent attempt (n = 86). Of the 86 participants, 41 (48%) had radiographic progression exceeding the smallest detectable change (5.7 units), and progression was associated with disease activity, not b/tsDMARD use.
Conclusion: Long-term disease activity-guided dose optimization of TNF inhibitors in RA, including discontinuation and multiple tapering attempts, remains safe and effective.
Keywords: TNF inhibitors; adalimumab; biologics; dose reduction; etanercept; rheumatoid arthritis; tapering.
© The Author(s) 2024. Published by Oxford University Press on behalf of the British Society for Rheumatology.
Figures
Similar articles
-
Down-titration and discontinuation strategies of tumour necrosis factor-blocking agents for rheumatoid arthritis in patients with low disease activity.Cochrane Database Syst Rev. 2019 May 24;5(5):CD010455. doi: 10.1002/14651858.CD010455.pub3. Cochrane Database Syst Rev. 2019. PMID: 31125448 Free PMC article.
-
Long-term outcomes after disease activity-guided dose reduction of TNF inhibition in rheumatoid arthritis: 3-year data of the DRESS study - a randomised controlled pragmatic non-inferiority strategy trial.Ann Rheum Dis. 2017 Oct;76(10):1716-1722. doi: 10.1136/annrheumdis-2017-211169. Epub 2017 Jun 12. Ann Rheum Dis. 2017. PMID: 28606961 Clinical Trial.
-
Which factors influence radiographic progression during treatment with tumor necrosis factor inhibitors in clinical practice? Results from 930 patients with rheumatoid arthritis in the nationwide Danish DANBIO registry.J Rheumatol. 2014 Dec;41(12):2352-60. doi: 10.3899/jrheum.131299. Epub 2014 Oct 1. J Rheumatol. 2014. PMID: 25274894
-
A multi-biomarker score measuring disease activity in rheumatoid arthritis patients tapering adalimumab or etanercept: predictive value for clinical and radiographic outcomes.Rheumatology (Oxford). 2017 Jun 1;56(6):973-980. doi: 10.1093/rheumatology/kex003. Rheumatology (Oxford). 2017. PMID: 28339738 Clinical Trial.
-
Structural joint damage and hand bone loss in patients with rheumatoid arthritis.Dan Med J. 2018 Mar;65(3):B5452. Dan Med J. 2018. PMID: 29510810 Review.
References
-
- Smolen JS, Landewé RBM, Bergstra SA et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2022 update. Ann Rheum Dis 2023;82:3–18. - PubMed
-
- Sepriano A, Kerschbaumer A, Bergstra SA et al. Safety of synthetic and biological DMARDs: a systematic literature review informing the 2022 update of the EULAR recommendations for the management of rheumatoid arthritis. Ann Rheum Dis 2023;82:107–18. - PubMed
-
- John M. Eisenberg Center for Clinical Decisions and Communications Science. Medicines for rheumatoid arthritis: a review of the research for adults. 2005. https://www.ncbi.nlm.nih.gov/books/NBK115126/table/consra2.tu2/ (1 September 2023, date last accessed). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

