Establishment of immune suppression by cancer cells in the tumor microenvironment
- PMID: 38346752
- PMCID: PMC10978970
- DOI: 10.2183/pjab.100.005
Establishment of immune suppression by cancer cells in the tumor microenvironment
Erratum in
-
Erratum to "Establishment of immune suppression by cancer cells in the tumor microenvironment".Proc Jpn Acad Ser B Phys Biol Sci. 2024;100(3):252. doi: 10.2183/pjab.100.016. Proc Jpn Acad Ser B Phys Biol Sci. 2024. PMID: 38462503 Free PMC article. No abstract available.
Abstract
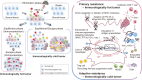
With the clinical success of immune checkpoint inhibitors (ICIs), cancer immunotherapy has become an important pillar of cancer treatment in various types of cancer. However, more than half of patients fail to respond to ICIs, even in combination, uncovering a limited window of clinical responses. Therefore, it is essential to develop more effective cancer immunotherapies and to define biomarkers for stratifying responders and nonresponders by exploring the immunological landscape in the tumor microenvironment (TME). It has become clear that differences in immune responses in the TME determine the clinical efficacy of cancer immunotherapies. Additionally, gene alterations in cancer cells contribute to the development of the immunological landscape, particularly immune suppression in the TME. Therefore, integrated analyses of immunological and genomic assays are key for understanding diverse immune suppressive mechanisms in the TME. Developing novel strategies to control immune suppression in the TME from the perspective of immunology and the cancer genome is crucial for effective cancer immunotherapy (immune-genome precision medicine).
Keywords: cancer immunotherapy; immune checkpoint inhibitors; immune suppression; immune-genome precision medicine; tumor microenvironment.
Conflict of interest statement
H.N. received research funding and honoraria from Ono Pharmaceutical, MSD, Bristol Myers Squibb, and Chugai Pharmaceutical, honoraria from Amgen, and research funding from Taiho Pharmaceutical, Daiichi-Sankyo, Kyowa Kirin, Zenyaku Kogyo, Oncolys BioPharma, Debiopharma, Asahi-Kasei, Sysmex, Fujifilm, SRL, Astellas Pharmaceutical, Sumitomo Dainippon Pharma, and BD Japan outside of this study. He serves as a board member as a founder of Sustainable Cell Therapeutics and Cellian-Biclo outside of this study.
Figures


References
-
- Kantoff P.W., Higano C.S., Shore N.D., Berger E.R., Small E.J., Penson D.F., et al. (2010) Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med. 363, 411–422. - PubMed

