Effects of metformin on glucose metabolism and mitochondrial function in patients with obstructive sleep apnea: A pilot randomized trial
- PMID: 38346816
- PMCID: PMC10861357
- DOI: 10.14814/phy2.15948
Effects of metformin on glucose metabolism and mitochondrial function in patients with obstructive sleep apnea: A pilot randomized trial
Abstract
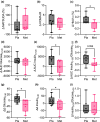
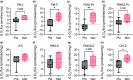
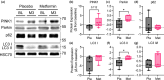
Obstructive sleep apnea (OSA) is associated with increased risk for diabetes, and standard treatment with positive airway pressure (PAP) device shows inconsistent effects on glucose metabolism. Metformin is known to treat and prevent diabetes, but its effects on skeletal muscle mitochondrial function are not completely understood. Here, we evaluate the effects of metformin on glucose metabolism and skeletal muscle mitochondrial function in patients with OSA. Sixteen adults with obesity (50.9 ± 6.7 years, BMI: 36.5 ± 2.9 kg/m2 ) and moderate-to-severe OSA were provided with PAP treatment and randomized to 3 months of placebo (n = 8) or metformin (n = 8) treatment in a double-blind parallel-group design. Whole body glucose metabolism was determined by oral glucose tolerance test. A skeletal muscle biopsy was obtained to evaluate mitochondrial respiratory capacity and expression of proteins related to mitochondrial dynamics and energy metabolism. Whole body insulin-sensitivity (Matsuda index) did not change in metformin or placebo treated groups. However, metformin treatment prevented increases in insulin release relative to placebo during follow-up. Insulin area under the curve (AUC) and insulin to glucose AUC ratio increased in placebo but remained unchanged with metformin. Furthermore, metformin treatment improved skeletal muscle mitochondrial respiratory capacity and dynamics relative to placebo. Metformin treatment prevented the decline in whole body glucose homeostasis and skeletal muscle mitochondrial function in patients with moderate to severe OSA. Patients with OSA may benefit from the addition of metformin to prevent diabetes.
Keywords: insulin sensitivity; metformin; mitochondrial function; obstructive sleep apnea.
© 2024 The Authors. Physiological Reports published by Wiley Periodicals LLC on behalf of The Physiological Society and the American Physiological Society.
Conflict of interest statement
The authors have nothing to disclose.
Figures



References
-
- Aroda, V. R. , Knowler, W. C. , Crandall, J. P. , Perreault, L. , Edelstein, S. L. , Jeffries, S. L. , Molitch, M. E. , Pi‐Sunyer, X. , Darwin, C. , Heckman‐Stoddard, B. M. , Temprosa, M. , Kahn, S. E. , & Nathan, D. M. (2017). Metformin for diabetes prevention: Insights gained from the diabetes prevention program/diabetes prevention program outcomes study. Diabetologia, 60(9), 1601–1611. 10.1007/s00125-017-4361-9 - DOI - PMC - PubMed
-
- Axelrod, C. L. , Fealy, C. E. , Erickson, M. L. , Davuluri, G. , Fujioka, H. , Dantas, W. S. , Huang, E. , Pergola, K. , Mey, J. T. , King, W. T. , Mulya, A. , Hsia, D. , Burguera, B. , Tandler, B. , Hoppel, C. L. , & Kirwan, J. P. (2021). Lipids activate skeletal muscle mitochondrial fission and quality control networks to induce insulin resistance in humans. Metabolism, Clinical and Experimental, 121, 154803. 10.1016/j.metabol.2021.154803 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

