Four-year clinical update and treatment switching-adjusted outcomes with first-line nivolumab plus ipilimumab with chemotherapy for metastatic non-small cell lung cancer in the CheckMate 9LA randomized trial
- PMID: 38346853
- PMCID: PMC10862253
- DOI: 10.1136/jitc-2023-008189
Four-year clinical update and treatment switching-adjusted outcomes with first-line nivolumab plus ipilimumab with chemotherapy for metastatic non-small cell lung cancer in the CheckMate 9LA randomized trial
Abstract
Background: In CheckMate 9LA, nivolumab plus ipilimumab with chemotherapy prolonged overall survival (OS) versus chemotherapy regardless of tumor PD-L1 expression or histology. We report updated efficacy and safety in all randomized patients with a minimum 4-year follow-up and an exploratory treatment-switching adjustment analysis in all treated patients who received chemotherapy and subsequent immunotherapy.
Methods: Adults with stage IV/recurrent non-small cell lung cancer (NSCLC), no sensitizing EGFR/ALK alterations, and ECOG performance status ≤1 were randomized 1:1 to nivolumab 360 mg every 3 weeks plus ipilimumab 1 mg/kg every 6 weeks with chemotherapy (two cycles) or chemotherapy (four cycles, with optional maintenance pemetrexed for the nonsquamous population). Assessments included OS, progression-free survival, and objective response rate. Exploratory analyses included efficacy by tumor PD-L1 expression and histology and in patients who discontinued nivolumab plus ipilimumab with chemotherapy due to treatment-related adverse events (TRAEs), and a treatment-switching adjustment analysis using inverse probability of censoring weighting.
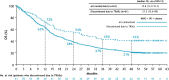
Results: With a 47.9-month minimum follow-up for OS, nivolumab plus ipilimumab with chemotherapy continued to prolong OS over chemotherapy in all randomized patients (HR 0.74, 95% CI 0.63 to 0.87; 4-year OS rate: 21% versus 16%), regardless of tumor PD-L1 expression (HR (95% CI): PD-L1<1%, 0.66 (0.50 to 0.86) and ≥1%, 0.74 (0.60 to 0.92)) or histology (squamous, 0.64 (0.48 to 0.84) and non-squamous, 0.80 (0.66 to 0.97)). In patients who discontinued all components of nivolumab plus ipilimumab with chemotherapy due to TRAEs (n=61), the 4-year OS rate was 41%. With treatment-switching adjustment for the 36% of patients receiving subsequent immunotherapy in the chemotherapy arm, the estimated HR of nivolumab plus ipilimumab with chemotherapy versus chemotherapy was 0.66 (95% CI 0.55 to 0.80). No new safety signals were observed.
Conclusions: In this 4-year update, patients treated with nivolumab plus ipilimumab with chemotherapy continued to have long-term, durable efficacy benefit over chemotherapy regardless of tumor PD-L1 expression and/or histology. A greater estimated relative OS benefit was observed after adjustment for subsequent immunotherapy use in the chemotherapy arm. These results further support nivolumab plus ipilimumab with chemotherapy as a first-line treatment for patients with metastatic/recurrent NSCLC, including those with tumor PD-L1<1% or squamous histology, populations with high unmet needs.
Keywords: clinical trials, phase III as topic; drug therapy, combination; immunotherapy; non-small cell lung cancer; programmed cell death 1 receptor.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: DPC has received consulting fees from Arcus Biosciences, Bristol Myers Squibb, BMS KK, Boehringer Ingelheim, Curio Science, Daiichi Sankyo, Genentech/Roche, GI Therapeutics (Intellisphere), GSK, Janssen, Merck, Mirati, Novartis, Novocure, OncoCyte, OncoHost, Roche China, and Seattle Genetics; and has participated on the advisory board of Amgen, Arcus Biosciences, AstraZeneca, Merck, Flame Biosciences, Gritstone Oncology, Cantargia (PPD), Daiichi Sankyo, EMD Serono GSK, Lilly, Regeneron, Sanofi, and Seattle Genetics and the data safety monitoring board of EORTC, AbbVie, and Lilly. T-EC has received honoraria from Astellas Pharma, Janssen, MSD, Merck Serono, Amgen, Roche, Pfizer, Sanofi Genzyme, Servier, Ipsen, AstraZeneca, Lilly, Novartis, Boehringer Ingelheim, and Bristol Myers Squibb; and has participated on a data safety monitoring board or advisory board of Astellas Pharma, Janssen, MSD, Merck Serono, Amgen, Roche, Pfizer, Sanofi Genzyme, Servier, Ipsen, AstraZeneca, Lilly, Novartis, Boehringer Ingelheim, and Bristol Myers Squibb. MS has received funding from Bristol Myers Squibb, MSD, Merck Serono, Pfizer, GSK, Roche, Bayer, Astellas, Amgen, Gilead, Tesaro, Clovis, Eli Lilly, Novartis, Regeneron, AbbVie, AstraZeneca, PharmaMar, Mylan, Samsung Pharmaceuticals, Bioven, BeiGene, and Daiichi Sankyo. OJ-V has received grants from AstraZeneca Spain, honoraria from Bristol Myers Squibb, Roche/Genentech, MSD Oncology, AstraZeneca/MedImmune, and Takeda; has served as a consultant for Bristol Myers Squibb, Lilly, Takeda, AstraZeneca Spain, and Janssen Oncology, and has received travel support from Takeda, AstraZeneca/MedImmune. NR has received honoraria from AstraZeneca, Bristol Myers Squibb, Boehringer Ingelheim, Daiichi Sankyo, GSK, Hoffmann-La Roche, Janssen, Lilly, MSD, Merck, Pfizer, Symphogen, and Takeda; travel support from AstraZeneca, Bristol Myers Squibb, Boehringer Ingelheim, Janssen, Hoffmann-La Roche, and Takeda; and has participated on a data safety monitoring board or advisory board for Merck and Symphogen. BZ has received honoraria from Amgen, AstraZeneca, Bristol Myers Squibb, GSK, Janssen-Cilag, MSD and Roche. AA has received consulting fees from Boehringer Ingelheim and Roche, provided expert testimony for Bristol Myers Squibb, Novartis, and Sandoz, and received travel support from AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Pfizer, Roche, and Sanofi. LP-A has received grants or contracts from MSD, AstraZeneca, Pfizer, and Bristol Myers Squibb; consulting fees from Lilly, MSD, Roche, PharmaMar, Merck KGaA (Darmstadt, Germany), AstraZeneca, Novartis, Servier, Amgen, Pfizer, Sanofi, Bayer, Bristol Myers Squibb, Mirati, GSK, Janssen, Takeda, and Daichii Sankyo; honoraria from AstraZeneca, Janssen, Merck, and Mirati; and has participated on a data safety monitoring board or advisory board for Altum Sequencing and Genomica. SL has received consulting fees from AstraZeneca, Boehringer Ingelheim, Hutchinson MediPharma, Simcere, ZaiLab, GenomiCare, Roche, and Hanosh, and honoraria from AstraZeneca, Roche, and Hanosh. TJ has received consulting fees from Roche, Merck, MSD, Puma, AstraZeneca, Bristol Myers Squibb, Amgen, Gilead, and Specialised Therapeutics; and honoraria from AstraZeneca. XZ holds stock in Bristol Myers Squibb. IS has received consulting fees from Bristol Myers Squibb. JRP holds stock in Bristol Myers Squibb. AL holds stock in Bristol Myers Squibb. MR has received consulting fees from Amgen, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, BeiGene, Lilly, Mirati, MSD, Merck, Novartis, Pfizer, Sanofi, Regeneron, Roche, Takeda, and Samsung Bioepis; honoraria from Amgen, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, BeiGene, Lilly, Mirati, MSD, Merck, Novartis, Pfizer, Sanofi, Regeneron, Roche, Takeda, and Samsung Bioepis; travel support from Amgen, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, BeiGene, Lilly, Mirati, MSD, Merck, Novartis, Pfizer, Sanofi, Regeneron, Roche, Takeda, and Samsung Bioepis; and has participated on a data safety monitoring board or advisory board for Amgen, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, BeiGene, Lilly, Mirati, MSD, Merck, Novartis, Pfizer, Sanofi, Regeneron, Roche, Takeda, and Samsung Bioepis. All other authors declare no competing interests.
Figures


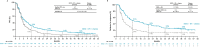

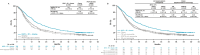
References
-
- Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) for Non-Small Cell Lung Cancer V5.2023. National Comprehensive Cancer Network, Inc . All rights reserved. Accessed November 16, 2023. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way. 2023.
-
- Paz-Ares LG, Ciuleanu T-E, Cobo M, et al. . First-line nivolumab plus ipilimumab with chemotherapy versus chemotherapy alone for metastatic NSCLC in CheckMate 9LA: 3-year clinical update and outcomes in patients with brain metastases or select somatic mutations. J Thorac Oncology 2023;18:204–22. 10.1016/j.jtho.2022.10.014 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
