Exploiting epigenetic targets to overcome taxane resistance in prostate cancer
- PMID: 38346967
- PMCID: PMC10861560
- DOI: 10.1038/s41419-024-06422-1
Exploiting epigenetic targets to overcome taxane resistance in prostate cancer
Abstract
The development of taxane resistance remains a major challenge for castration resistant prostate cancer (CR-PCa), despite the effectiveness of taxanes in prolonging patient survival. To uncover novel targets, we performed an epigenetic drug screen on taxane (docetaxel and cabazitaxel) resistant CR-PCa cells. We identified BRPF reader proteins, along with several epigenetic groups (CBP/p300, Menin-MLL, PRMT5 and SIRT1) that act as targets effectively reversing the resistance mediated by ABCB1. Targeting BRPFs specifically resulted in the resensitization of resistant cells, while no such effect was observed on the sensitive compartment. These cells were successfully arrested at the G2/M phase of cell cycle and underwent apoptosis upon BRPF inhibition, confirming the restoration of taxane susceptibility. Pharmacological inhibition of BRPFs reduced ABCB1 activity, indicating that BRPFs may be involved in an efflux-related mechanism. Indeed, ChIP-qPCR analysis confirmed binding of BRPF1 to the ABCB1 promoter suggesting direct regulation of the ABCB1 gene at the transcriptional level. RNA-seq analysis revealed that BRPF1 knockdown affects the genes enriched in mTORC1 and UPR signaling pathways, revealing potential mechanisms underlying its functional impact, which is further supported by the enhancement of taxane response through the combined inhibition of ABCB1 and mTOR pathways, providing evidence for the involvement of multiple BRPF1-regulated pathways. Beyond clinical attributes (Gleason score, tumor stage, therapy outcome, recurrence), metastatic PCa databases further supported the significance of BRPF1 in taxane resistance, as evidenced by its upregulation in taxane-exposed PCa patients.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

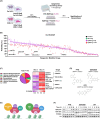
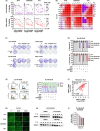
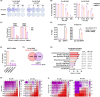
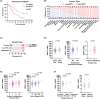
References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J Clin. 2018;68:394–424. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

