Poly-γ-glutamylation of biomolecules
- PMID: 38346985
- PMCID: PMC10861534
- DOI: 10.1038/s41467-024-45632-1
Poly-γ-glutamylation of biomolecules
Abstract
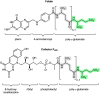
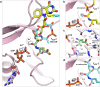
Poly-γ-glutamate tails are a distinctive feature of archaeal, bacterial, and eukaryotic cofactors, including the folates and F420. Despite decades of research, key mechanistic questions remain as to how enzymes successively add glutamates to poly-γ-glutamate chains while maintaining cofactor specificity. Here, we show how poly-γ-glutamylation of folate and F420 by folylpolyglutamate synthases and γ-glutamyl ligases, non-homologous enzymes, occurs via processive addition of L-glutamate onto growing γ-glutamyl chain termini. We further reveal structural snapshots of the archaeal γ-glutamyl ligase (CofE) in action, crucially including a bulged-chain product that shows how the cofactor is retained while successive glutamates are added to the chain terminus. This bulging substrate model of processive poly-γ-glutamylation by terminal extension is arguably ubiquitous in such biopolymerisation reactions, including addition to folates, and demonstrates convergent evolution in diverse species from archaea to humans.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

