Digital health technologies and machine learning augment patient reported outcomes to remotely characterise rheumatoid arthritis
- PMID: 38347090
- PMCID: PMC10861520
- DOI: 10.1038/s41746-024-01013-y
Digital health technologies and machine learning augment patient reported outcomes to remotely characterise rheumatoid arthritis
Abstract
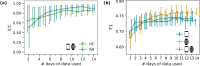
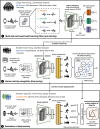
Digital measures of health status captured during daily life could greatly augment current in-clinic assessments for rheumatoid arthritis (RA), to enable better assessment of disease progression and impact. This work presents results from weaRAble-PRO, a 14-day observational study, which aimed to investigate how digital health technologies (DHT), such as smartphones and wearables, could augment patient reported outcomes (PRO) to determine RA status and severity in a study of 30 moderate-to-severe RA patients, compared to 30 matched healthy controls (HC). Sensor-based measures of health status, mobility, dexterity, fatigue, and other RA specific symptoms were extracted from daily iPhone guided tests (GT), as well as actigraphy and heart rate sensor data, which was passively recorded from patients' Apple smartwatch continuously over the study duration. We subsequently developed a machine learning (ML) framework to distinguish RA status and to estimate RA severity. It was found that daily wearable sensor-outcomes robustly distinguished RA from HC participants (F1, 0.807). Furthermore, by day 7 of the study (half-way), a sufficient volume of data had been collected to reliably capture the characteristics of RA participants. In addition, we observed that the detection of RA severity levels could be improved by augmenting standard patient reported outcomes with sensor-based features (F1, 0.833) in comparison to using PRO assessments alone (F1, 0.759), and that the combination of modalities could reliability measure continuous RA severity, as determined by the clinician-assessed RAPID-3 score at baseline (r2, 0.692; RMSE, 1.33). The ability to measure the impact of the disease during daily life-through objective and remote digital outcomes-paves the way forward to enable the development of more patient-centric and personalised measurements for use in RA clinical trials.
© 2024. The Author(s).
Conflict of interest statement
A.P.C, H.Y, G.M, A.D, D.A.C are employees of the University of Oxford. A.P.C is a GSK postdoctoral fellow and acknowledges the support of GSK. D.A.C received research funding from GSK to conduct this work. In addition, A.D., H.Y., and G.M. acknowledge the support of Novo Nordisk plc. A.D. AD is supported by the Wellcome Trust [223100/Z/21/Z]. V.H, W-H.C, R.T, R.W and L.G-G are employees of GSK and own stock and or shares. C.L, C.Y, M.S.D are employees of Analysis Group, which received research funding from GSK to conduct the study.
Figures


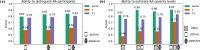
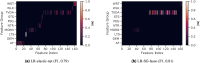

References
-
- Campbell, R., Ju, A., King, M. T. & Rutherford, C. Perceived benefits and limitations of using patient-reported outcome measures in clinical practice with individual patients: a systematic review of qualitative studies. Quality Life Res. 1–24 (2021). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

