Discovery of dual kinase inhibitors targeting VEGFR2 and FAK: structure-based pharmacophore modeling, virtual screening, and molecular docking studies
- PMID: 38347617
- PMCID: PMC10863211
- DOI: 10.1186/s13065-024-01130-5
Discovery of dual kinase inhibitors targeting VEGFR2 and FAK: structure-based pharmacophore modeling, virtual screening, and molecular docking studies
Abstract
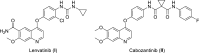
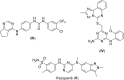
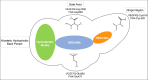
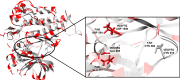
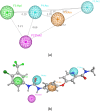
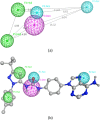
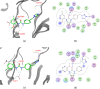
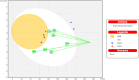
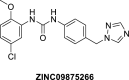
VEGFR2 and FAK signaling pathways are interconnected and have synergistic effects on tumor angiogenesis, growth, and metastasis. Thus, instead of the conventional targeting of each of these proteins individually with a specific inhibitor, the present work aimed to discover novel dual inhibitors targeting both VEGFR2 and FAK exploiting their association. To this end, receptor-based pharmacophore modeling technique was opted to generate 3D pharmacophore models for VEGFR2 and FAK type II kinase inhibitors. The generated pharmacophore models were validated by assessing their ability to discriminate between active and decoy compounds in a pre-compiled test set of VEGFR2 and FAK active compounds and decoys. ZINCPharmer web tool was then used to screen the ZINC database purchasable subset using the validated pharmacophore models retrieving 42,616 hits for VEGFR2 and 28,475 hits for FAK. Subsequently, they were filtered using various filters leaving 13,023 and 6,832 survived compounds for VEGFR2 and FAK, respectively, with 124 common compounds. Based on molecular docking simulations, thirteen compounds were found to satisfy all necessary interactions with VEGFR2 and FAK kinase domains. Thus, they are predicted to have a possible dual VEGFR2/FAK inhibitory activity. Finally, SwissADME web tool showed that compound ZINC09875266 is not only promising in terms of binding pattern to our target kinases, but also in terms of pharmacokinetic properties.
Keywords: Angiogenesis; Cancer; FAK; Molecular docking; Multi-kinase inhibitors; Pharmacophore modelling; VEGFR2; Virtual screening.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Receptor-based pharmacophore modeling, virtual screening, and molecular docking studies for the discovery of novel GSK-3β inhibitors.J Mol Model. 2019 May 25;25(6):171. doi: 10.1007/s00894-019-4032-5. J Mol Model. 2019. PMID: 31129879
-
Molecular dynamics guided insight, binding free energy calculations and pharmacophore-based virtual screening for the identification of potential VEGFR2 inhibitors.J Recept Signal Transduct Res. 2019 Oct-Dec;39(5-6):415-433. doi: 10.1080/10799893.2019.1690509. Epub 2019 Nov 22. J Recept Signal Transduct Res. 2019. PMID: 31755336
-
Ligand-Based Pharmacophore Modeling, Molecular Docking, and Molecular Dynamic Studies of Dual Tyrosine Kinase Inhibitor of EGFR and VEGFR2.Int J Mol Sci. 2020 Oct 21;21(20):7779. doi: 10.3390/ijms21207779. Int J Mol Sci. 2020. PMID: 33096664 Free PMC article.
-
Computational insights into allosteric inhibition of focal adhesion kinase: A combined pharmacophore modeling and molecular dynamics approach.J Mol Graph Model. 2024 Jul;130:108789. doi: 10.1016/j.jmgm.2024.108789. Epub 2024 May 4. J Mol Graph Model. 2024. PMID: 38718434
-
Identification of potentially high drug-like VEGFR2/c-Met dual-target type II kinase inhibitors with symmetric skeletons based on structural screening.J Biomol Struct Dyn. 2024 Feb-Mar;42(3):1249-1267. doi: 10.1080/07391102.2023.2199082. Epub 2023 Apr 12. J Biomol Struct Dyn. 2024. PMID: 37042992
Cited by
-
Advances in VEGFR Inhibitors: A Comprehensive Review of Novel Anticancer Agents.Anticancer Agents Med Chem. 2025;25(10):663-687. doi: 10.2174/0118715206356712241202112641. Anticancer Agents Med Chem. 2025. PMID: 39810521 Review.
-
Discovery of Vascular Endothelial Growth Factor Receptor 2 Inhibitors Employing Junction Tree Variational Autoencoder with Bayesian Optimization and Gradient Ascent.ACS Omega. 2024 Nov 12;9(47):47180-47193. doi: 10.1021/acsomega.4c07689. eCollection 2024 Nov 26. ACS Omega. 2024. PMID: 39619551 Free PMC article.
-
Potential VEGFR2 inhibitors for managing metastatic cervical cancer: insights from molecular dynamics and free energy landscape studies.Mol Divers. 2024 Dec 18. doi: 10.1007/s11030-024-11080-8. Online ahead of print. Mol Divers. 2024. PMID: 39693033
References
LinkOut - more resources
Full Text Sources
Miscellaneous
